Question
Consider the following reactions and their heats of combustion. Compound Combustion Reaction 1-butene HC=CHCHCH +604CO+ 4HO CH3 cis- 2-butene trans-2- butene 2-methyl-1- propene H3C,
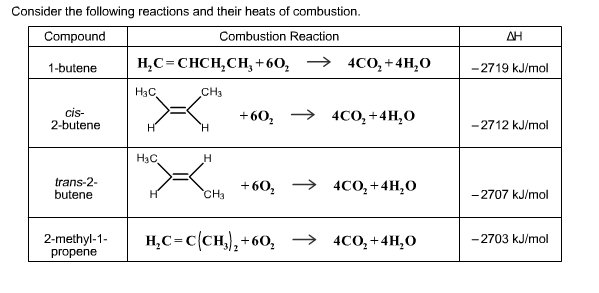
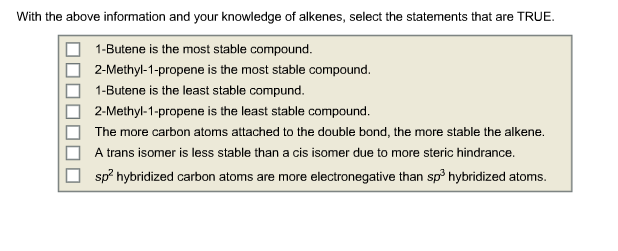
Consider the following reactions and their heats of combustion. Compound Combustion Reaction 1-butene HC=CHCHCH +604CO+ 4HO CH3 cis- 2-butene trans-2- butene 2-methyl-1- propene H3C, H HC H H H CH3 +60 4CO + 4HO +60 4CO+ 4HO HC=C(CH)+60 4CO+ 4HO -2719 kJ/mol -2712 kJ/mol -2707 kJ/mol -2703 kJ/mol With the above information and your knowledge of alkenes, select the statements that are TRUE. 1-Butene is the most stable compound. 2-Methyl-1-propene is the most stable compound. 1-Butene is the least stable compund. 2-Methyl-1-propene is the least stable compound. The more carbon atoms attached to the double bond, the more stable the alkene. A trans isomer is less stable than a cis isomer due to more steric hindrance. sp hybridized carbon atoms are more electronegative than sp hybridized atoms.
Step by Step Solution
3.42 Rating (142 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Chemical Principles
Authors: Steven S. Zumdahl, Donald J. DeCoste
7th edition
9781133109235, 1111580650, 978-1111580650
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



