Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Consider the following system of gas-phase reactions: AXABAYrX=k1CA1/2rB=k2CArY=k3CA2k1=0.004(mol/dm3)1/2min1k2=0.3min1k3=0.25dm3/molmin B is the desired product, and X and Y are foul pollutants that are expensive to get
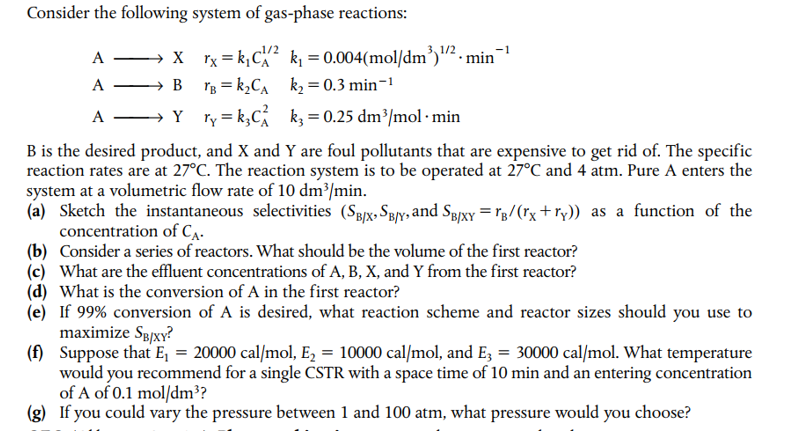 Consider the following system of gas-phase reactions: AXABAYrX=k1CA1/2rB=k2CArY=k3CA2k1=0.004(mol/dm3)1/2min1k2=0.3min1k3=0.25dm3/molmin B is the desired product, and X and Y are foul pollutants that are expensive to get rid of. The specific reaction rates are at 27C. The reaction system is to be operated at 27C and 4atm. Pure A enters the system at a volumetric flow rate of 10dm3/min. (a) Sketch the instantaneous selectivities (SB/X,SB/Y, and SB/XY=rB/(rX+rY)) as a function of the concentration of CA. (b) Consider a series of reactors. What should be the volume of the first reactor? (c) What are the effluent concentrations of A,B,X, and Y from the first reactor? (d) What is the conversion of A in the first reactor? (e) If 99% conversion of A is desired, what reaction scheme and reactor sizes should you use to maximize SB/XY ? (f) Suppose that E1=20000cal/mol,E2=10000cal/mol, and E3=30000cal/mol. What temperature would you recommend for a single CSTR with a space time of 10min and an entering concentration of A of 0.1mol/dm3 ? (g) If you could vary the pressure between 1 and 100atm, what pressure would you choose
Consider the following system of gas-phase reactions: AXABAYrX=k1CA1/2rB=k2CArY=k3CA2k1=0.004(mol/dm3)1/2min1k2=0.3min1k3=0.25dm3/molmin B is the desired product, and X and Y are foul pollutants that are expensive to get rid of. The specific reaction rates are at 27C. The reaction system is to be operated at 27C and 4atm. Pure A enters the system at a volumetric flow rate of 10dm3/min. (a) Sketch the instantaneous selectivities (SB/X,SB/Y, and SB/XY=rB/(rX+rY)) as a function of the concentration of CA. (b) Consider a series of reactors. What should be the volume of the first reactor? (c) What are the effluent concentrations of A,B,X, and Y from the first reactor? (d) What is the conversion of A in the first reactor? (e) If 99% conversion of A is desired, what reaction scheme and reactor sizes should you use to maximize SB/XY ? (f) Suppose that E1=20000cal/mol,E2=10000cal/mol, and E3=30000cal/mol. What temperature would you recommend for a single CSTR with a space time of 10min and an entering concentration of A of 0.1mol/dm3 ? (g) If you could vary the pressure between 1 and 100atm, what pressure would you choose Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


