Question
Consider the ideal gas equation of state: PV = mRT = nRT R where R = , R is universal gas constant = 8.314
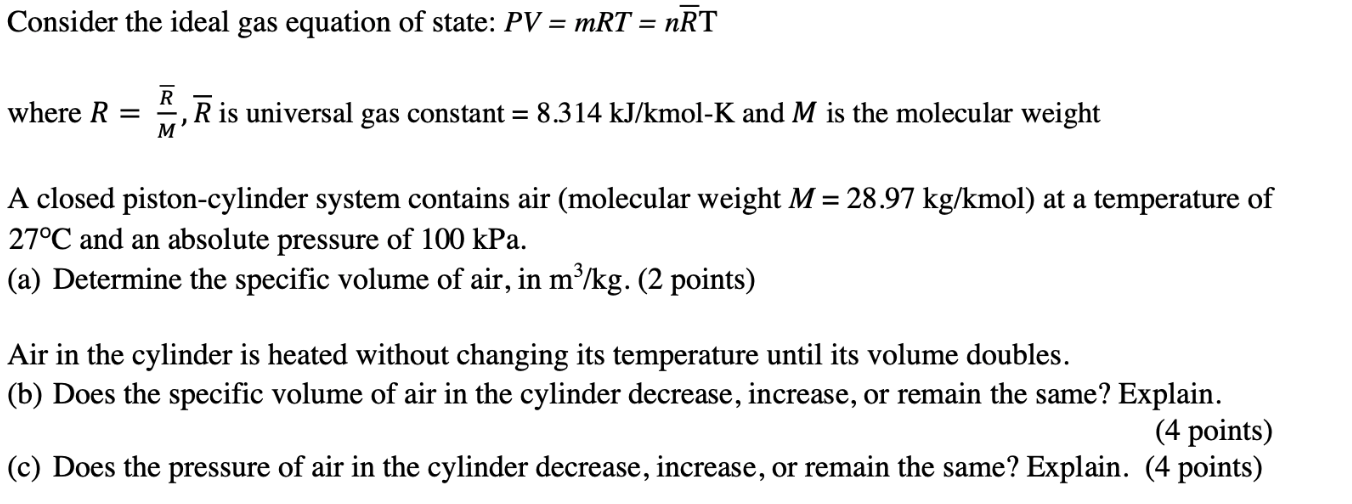
Consider the ideal gas equation of state: PV = mRT = nRT R where R = , R is universal gas constant = 8.314 kJ/kmol-K and M is the molecular weight M' A closed piston-cylinder system contains air (molecular weight M = 28.97 kg/kmol) at a temperature of 27C and an absolute pressure of 100 kPa. (a) Determine the specific volume of air, in m/kg. (2 points) Air in the cylinder is heated without changing its temperature until its volume doubles. (b) Does the specific volume of air in the cylinder decrease, increase, or remain the same? Explain. (4 points) (c) Does the pressure of air in the cylinder decrease, increase, or remain the same? Explain. (4 points)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Elementary Principles of Chemical Processes
Authors: Richard M. Felder, Ronald W. Rousseau
3rd Edition
978-0471687573, 9788126515820, 978-0-471-4152, 0471720631, 047168757X, 8126515821, 978-0471720638
Students also viewed these Mechanical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



