Question: Consider these steps from the recent literature and answer the questions that follow. i. Briefly explain what is happening at each step (i)(iv). 2 marks
Consider these steps from the recent literature and answer the questions that follow.
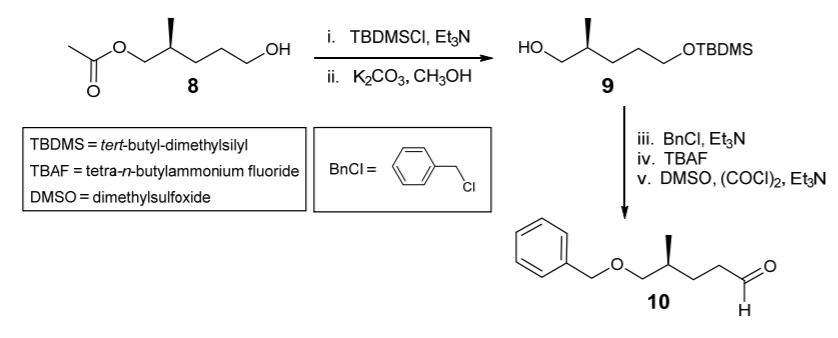
i. Briefly explain what is happening at each step (i)–(iv). 2 marks
ii. By considering the acetate (CH3CO), tert-butyldimethylsilyl (TBDMS) and benzyl (Bn or PhCH2) groups used in this example, explain what is meant by an orthogonal protecting group strategy. 2 marks
iii. Propose a mechanism for the transesterification reaction occurring in step (ii). 2 marks
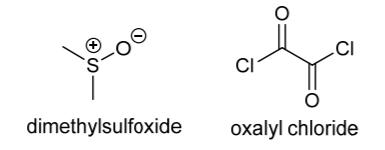
iv. Outline a mechanism to show how dimethylsulfoxide (DMSO) and oxalyl chloride can combine to generate an activated sulfur-centred electrophile, while liberating CO2, CO and chloride ion (the first stage of step (v)). 3 marks
i. TBDMSCI, Et,N HO . COTBDMS ii. KCO3, CH;OH 8 iii. BNCI, Et,N iv. TBAF v. DMSO, (COCI)2, EtN TBDMS = tert-butyl-dimethylsilyl TBAF = tetra-n-butylammonium fluoride BnCI = CI DMSO = dimethylsulfoxide 10 H
Step by Step Solution
3.41 Rating (148 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


