Question: Consider these steps the recent literature and answer the questions that follow. i. Propose a mechanism for step (i), formation of the thioacetal 12. 3
Consider these steps the recent literature and answer the questions that follow.
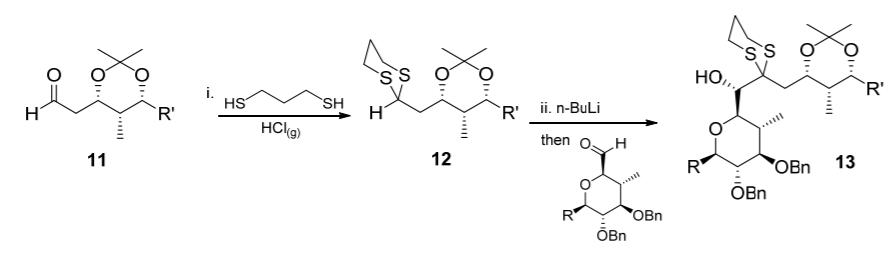
i. Propose a mechanism for step (i), formation of the thioacetal 12. 3 marks
ii. Propose a mechanism for step (ii). (Note that n-BuLi is a strong base, and assume that the alkoxide intermediate is protonated upon workup.) 2 marks
iii. Draw the synthon corresponding to aldehyde 11 in step (i), and the synthon corresponding to dithiane 12 in step (ii). 2 marks
iv. Building on your answer to part (iii), compare the reactivity of aldehyde 11 and dithiane 12 to explain the concept of umpolung. 2 marks
HO, R' i. HS `R' SH H H `R' ii. n-BuLi HCK9) then o H 11 12 R' OBn 13 Bn OBn Bn O..
Step by Step Solution
3.46 Rating (149 Votes )
There are 3 Steps involved in it
Detailed m... View full answer

Get step-by-step solutions from verified subject matter experts


