Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Consider these two expressions for the total molar flux of Neon in a multi-component mixture (note that Dve is an abbreviated version of DNe,mix):
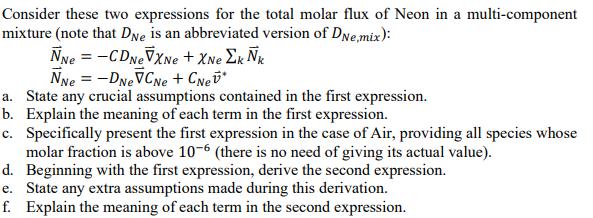
Consider these two expressions for the total molar flux of Neon in a multi-component mixture (note that Dve is an abbreviated version of DNe,mix): NNe = - CDNEVXne + XNe Ek k NNe = -DNEVCNe + CNet* a. State any crucial assumptions contained in the first expression. b. Explain the meaning of each term in the first expression. c. Specifically present the first expression in the case of Air, providing all species whose molar fraction is above 10-6 (there is no need of giving its actual value). Beginning with the first expression, derive the second expression. e. State any extra assumptions made during this derivation. d. f. Explain the meaning of each term in the second expression.
Step by Step Solution
There are 3 Steps involved in it
Step: 1
a The first expression assumes that the mixture is in local thermodynamic equilibrium LTE meaning that the temperatures and pressures of the component...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


