Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Determine the oxidation state of specific elements in the organic molecules. Note that each carbon can have up to four chemical bonds. In most
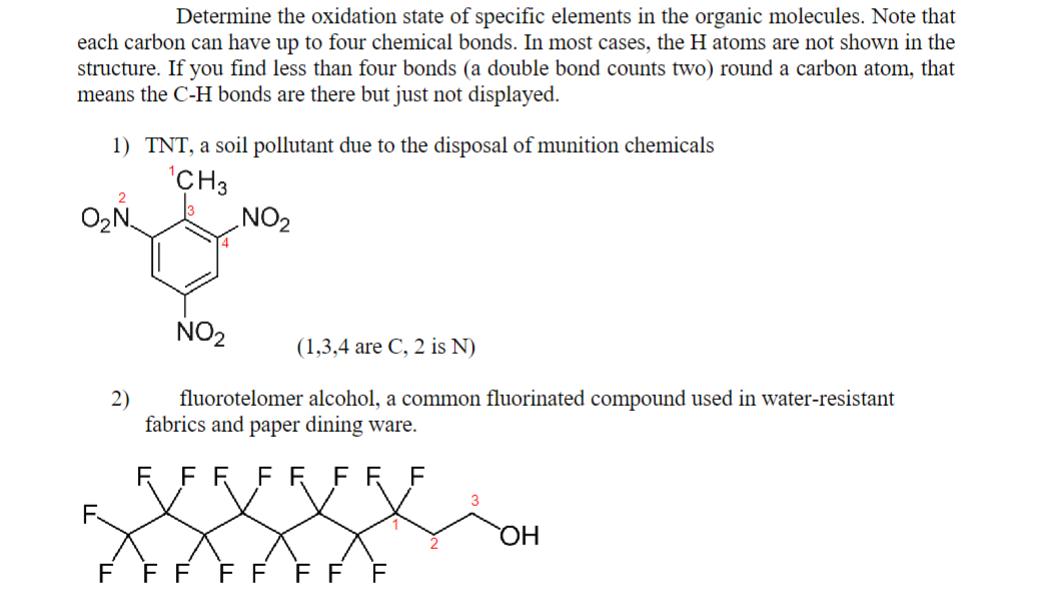
Determine the oxidation state of specific elements in the organic molecules. Note that each carbon can have up to four chemical bonds. In most cases, the H atoms are not shown in the structure. If you find less than four bonds (a double bond counts two) round a carbon atom, that means the C-H bonds are there but just not displayed. 1) TNT, a soil pollutant due to the disposal of munition chemicals CH3 ON. 2) NO NO (1,3,4 are C, 2 is N) fluorotelomer alcohol, a common fluorinated compound used in water-resistant fabrics and paper dining ware. F FR FR FFF F FF FF FF F OH Determine the oxidation state of specific elements in the organic molecules. Note that each carbon can have up to four chemical bonds. In most cases, the H atoms are not shown in the structure. If you find less than four bonds (a double bond counts two) round a carbon atom, that means the C-H bonds are there but just not displayed. 1) TNT, a soil pollutant due to the disposal of munition chemicals CH3 ON. 2) NO NO (1,3,4 are C, 2 is N) fluorotelomer alcohol, a common fluorinated compound used in water-resistant fabrics and paper dining ware. F FR FR FFF F FF FF FF F OH
Step by Step Solution
★★★★★
3.37 Rating (141 Votes )
There are 3 Steps involved in it
Step: 1
Here are the oxidation states of the elements in the two molecules ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


