Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The Haber Reaction produces ammonia NH3(g) from gaseous nitrogen and hydrogen: N(g) + 3H(g) 2NH3(g). It is one of the most important chemical reactions
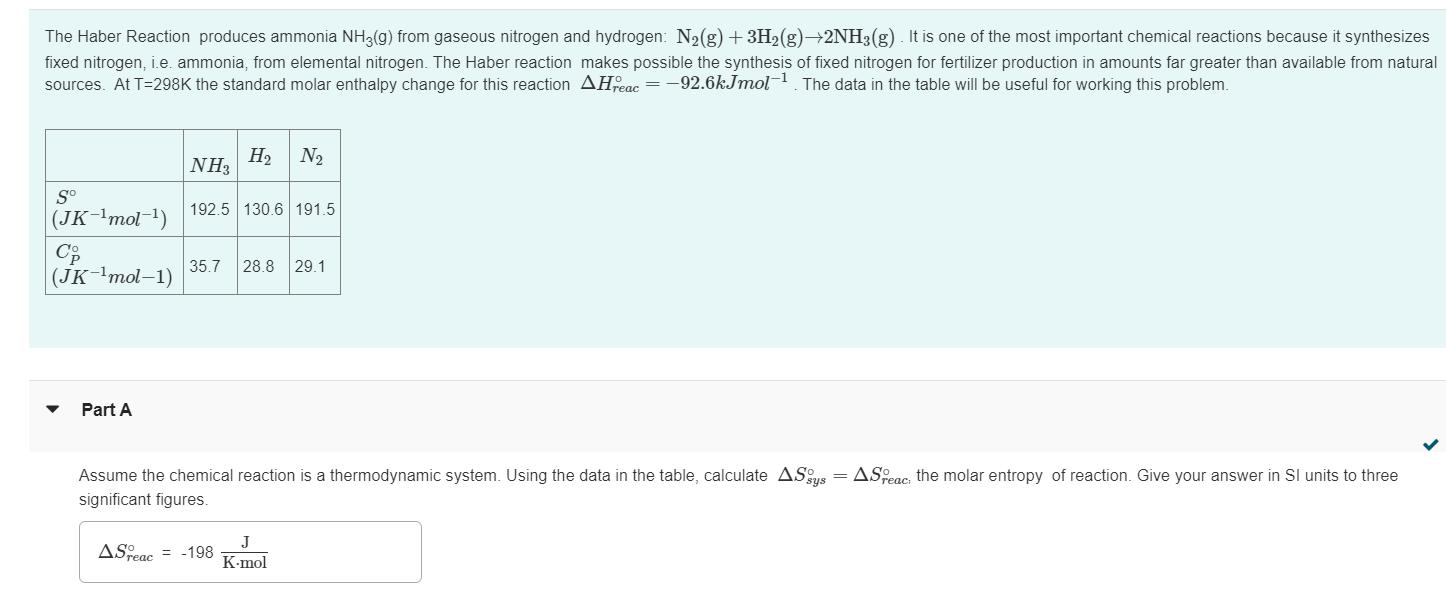

The Haber Reaction produces ammonia NH3(g) from gaseous nitrogen and hydrogen: N(g) + 3H(g) 2NH3(g). It is one of the most important chemical reactions because it synthesizes fixed nitrogen, i.e. ammonia, from elemental nitrogen. The Haber reaction makes possible the synthesis of fixed nitrogen for fertilizer production in amounts far greater than available from natural sources. At T=298K the standard molar enthalpy change for this reaction AHFeac=-92.6kJmol-. The data in the table will be useful for working this problem. So (JK-mol-) Co (JK-mol-1) Part A NH H N 192.5 130.6 191.5 35.7 28.8 29.1 Assume the chemical reaction is a thermodynamic system. Using the data in the table, calculate ASys = ASPeac: the molar entropy of reaction. Give your answer in SI units to three significant figures. ASreac = -198 J K-mol Can the Haber reaction be made more thermodynamically favorable by increasing the temperature? To explore this question use the data in the table to calculate ASreac, the molar entropy change of the reaction at T-500K. Assume all heat capacities are constant between T-298K and T-500K. ASTeac = -221 Submit J K-mol Previous Answers
Step by Step Solution
★★★★★
3.43 Rating (150 Votes )
There are 3 Steps involved in it
Step: 1
N 8 3 4 8 2NH3 8 pant E part F T 500k AH reac 2 926...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


