Question
Consider two bulbs containing different mixtures of gases and connected by a capillary as shown in the following figure. In bulb A the gas mixture
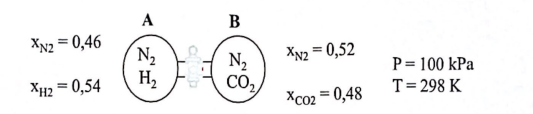
Consider two bulbs containing different mixtures of gases and connected by a capillary as shown in the following figure.
In bulb A the gas mixture is composed of nitrogen and hydrogen with mole fractions of 0.46 and 0.54, respectively. In bulb B the gas mixture is composed of nitrogen and carbon dioxide with mole fractions of 0.52 and 0.48, respectively. The temperature and pressure of the bulbs is identical and equal to 298 K and 100 kPa.
The volume of each bulb is 100 mL, the length and diameter of the capillary are 0.1 m and 0.001 m, respectively. Under the conditions of the experiment the binary diffusion coefficients are known:
DN2-H2=7.79.10-5 m/s Dco2-H2 6.46.10-5 m/s DN2-C02 1.65.105 m/s
Calculate the composition change in each bulb as a function of time. Also, graphically represent.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


