Question
Considering the following table of pK, values. PK values of organic and inorganic acids pKa Acid pK 51 38 CH3 CH3 NH3 H 35
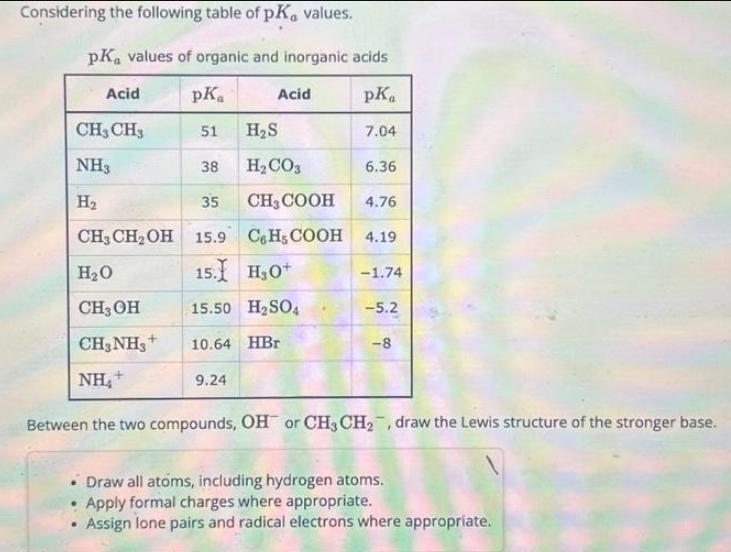
Considering the following table of pK, values. PK values of organic and inorganic acids pKa Acid pK 51 38 CH3 CH3 NH3 H 35 CH3 CHOH 15.9 HO Acid HS HCO3 CH3COOH 4.76 C6H5COOH 4.19 15. HO+ 15.50 HSO4 10.64 HBr 9.24 7.04 6.36 -1.74 CH3OH CH3NH+ NH+ Between the two compounds, OH or CH3 CH, draw the Lewis structure of the stronger base. -5.2 -8 Draw all atoms, including hydrogen atoms. Apply formal charges where appropriate. Assign lone pairs and radical electrons where appropriate.
Step by Step Solution
3.43 Rating (159 Votes )
There are 3 Steps involved in it
Step: 1
Solutions Step 1 Based on the pKa values provided OH is the stronger base compared to CHCH This can ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Introductory Chemical Engineering Thermodynamics
Authors: J. Elliott, Carl Lira
2nd Edition
0136068545, 978-0136068549
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



