Answered step by step
Verified Expert Solution
Question
1 Approved Answer
could you help asap please You are studying FeCl3 and you are asked to determine the Ksp expression in terms of a single variable along
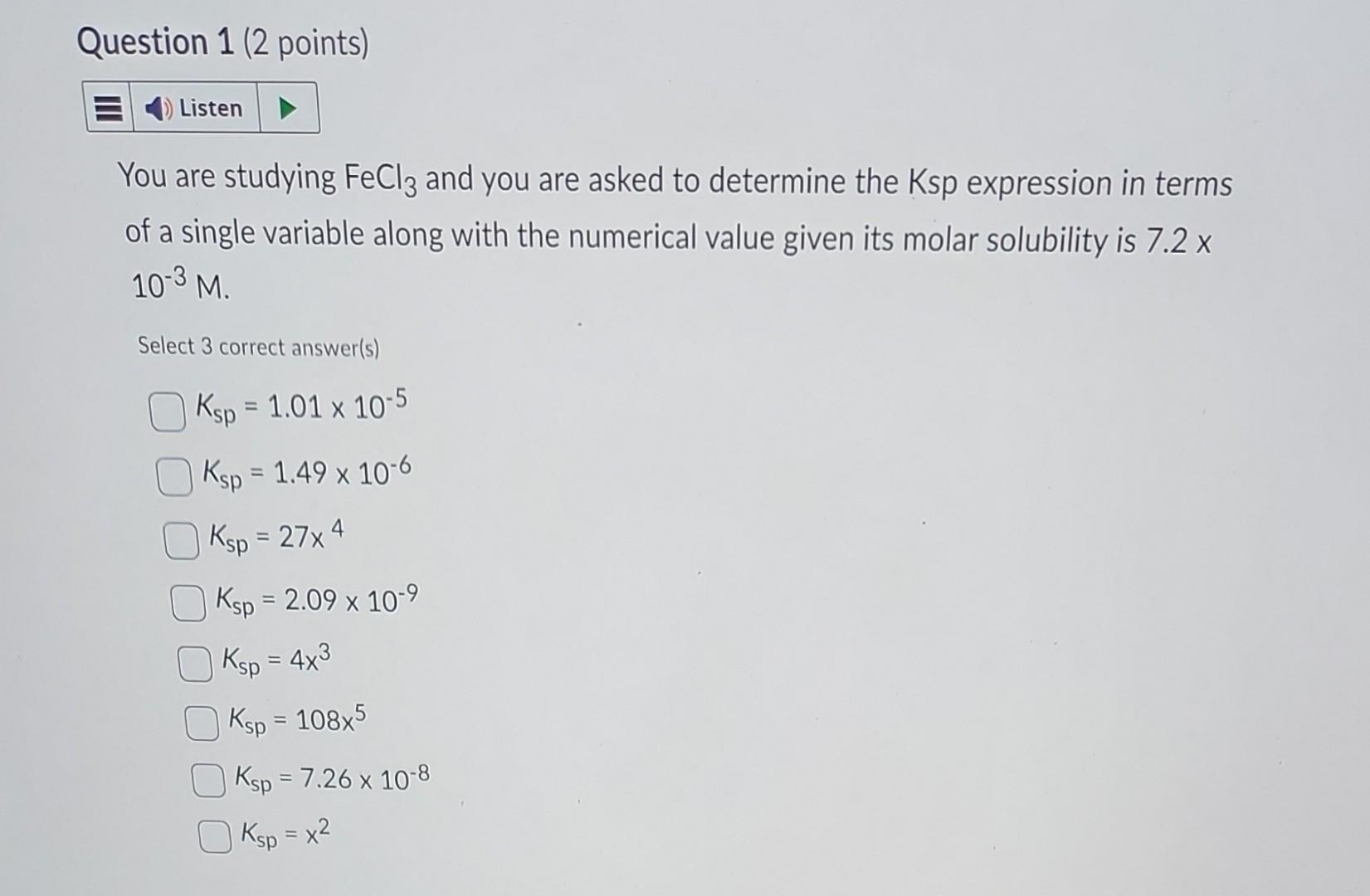


could you help asap please
You are studying FeCl3 and you are asked to determine the Ksp expression in terms of a single variable along with the numerical value given its molar solubility is 7.2x 103M. Select 3 correct answer(s) Ksp=1.01105Ksp=1.49106Ksp=27x4Ksp=2.09109Ksp=4x3Ksp=108x5Ksp=7.26108Ksp=x2 You need to convert 0.502g/20mL to g/100mL. What is the appropriate conversion? (20mL4)(0.502g4)=2.008g/100mL(20mL8)(0.502g8)=4.016g/100mL(20mL5)(0.502g5)=2.51g/100mL(20mL10)(0.502g10)=5.02g/100mL From your graph of temperature in degrees Celcius ( x axis) and molar solubility in g/1 L (y axis), you estimated your the molar solubility value at 40C to be 1.8106 (units) for PbCl2. Would the molar solubility in terms of Moles per liter for PbCl2 be 6.471013? MWPbCl2=278.1g/mol True FalseStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


