Answered step by step
Verified Expert Solution
Question
1 Approved Answer
deal gas equation:P.V=N.R.T a=12.55 b=0.002 Determining the amount of he methane fuel that can be held in a 3 cubic meter tank at a temperature
deal gas equation:P.V=N.R.T
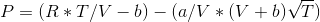
a=12.55
b=0.002
Determining the amount of he methane fuel that can be held in a 3 cubic meter tank at a temperature of -40 degree Celcius with pressure of 65 kPa.
write a c++ program to Use Newton-Raphson to calculate V and then determine the mass of the methane contained in the tank
could you write a program c++ using newton-Raphson Please?
P=(R*T/V - b) - (a/V *(V+b)VT) R=0,518 P= 4600 T = 191
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


