design the project

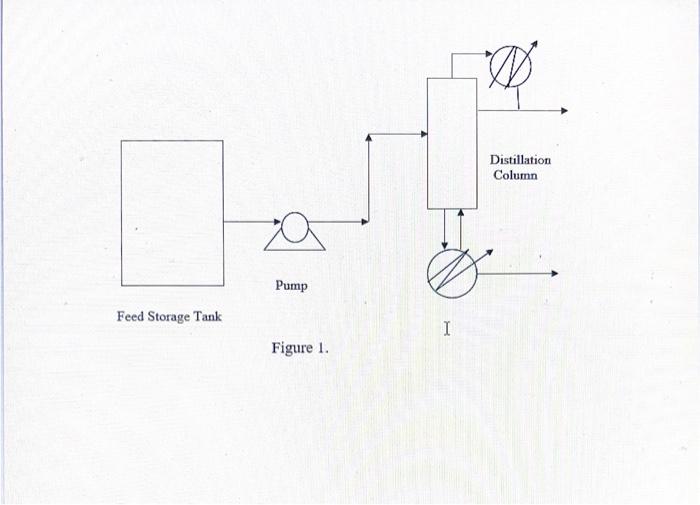
Figure 1 shows a unit for processing 1,000 kmole/hour of a mixture containing 30 mole% benzene (CH), and 70 mole% toluene (CH:CH:) at 20 C and 14.7 psia. The feed is pumped from the storage tank to a pressure of 40 psia and enters a distillation column where benzene is separated from toluene. The top product from the column contains 5 mole% toluene, and the bottom product contains 5 mole% benzene. McCabe-Thiele diagram for the column is given in Figure 2. Design the unit. Specify all your assumptions clearly. Available Data Average density of liquid benzene is 922 Kg/m! Average density of liquid toluene is 788 Kg/m Residence time in the storage tank is 5 days. Temperature of cooling water = 20 C. Steam at 200 C (with a heat of vaporization of 1,938.6 KJ/Kg) is available. Temperature at the top of column=80.1 C. Temperature at the bottom of column=110.8C. Overall heat transfer coefficient for condenser = 949 KJ/h m?.K. Overall heat transfer coefficient for reboiler = 1,265 KJh m?.K Optimum reflux ratio in distillation column is 2.68. CE cost index for 2022 is estimated to be 780 Heat of vaporization of mixture in the condenser is 31.3 KJ/mole Heat of vaporization of mixture in the reboiler is 38 KJ/mol Tray efficiency is 50% Pressure in the condenser is 35 psia Pressure in the reboiler is 45 psia Surface tension in the column is 28.6 dyne/cm Weight of the column heads is 10% of the weight of the shell Thickness of column (shell) = 1/4 inches Material construction: Carbon steel Tray spacing: 24 inches Density of carbon steel= 7,833 Kg/m Distillation Column Pump Feed Storage Tank I Figure 1. Figure 1 shows a unit for processing 1,000 kmole/hour of a mixture containing 30 mole% benzene (CH), and 70 mole% toluene (CH:CH:) at 20 C and 14.7 psia. The feed is pumped from the storage tank to a pressure of 40 psia and enters a distillation column where benzene is separated from toluene. The top product from the column contains 5 mole% toluene, and the bottom product contains 5 mole% benzene. McCabe-Thiele diagram for the column is given in Figure 2. Design the unit. Specify all your assumptions clearly. Available Data Average density of liquid benzene is 922 Kg/m! Average density of liquid toluene is 788 Kg/m Residence time in the storage tank is 5 days. Temperature of cooling water = 20 C. Steam at 200 C (with a heat of vaporization of 1,938.6 KJ/Kg) is available. Temperature at the top of column=80.1 C. Temperature at the bottom of column=110.8C. Overall heat transfer coefficient for condenser = 949 KJ/h m?.K. Overall heat transfer coefficient for reboiler = 1,265 KJh m?.K Optimum reflux ratio in distillation column is 2.68. CE cost index for 2022 is estimated to be 780 Heat of vaporization of mixture in the condenser is 31.3 KJ/mole Heat of vaporization of mixture in the reboiler is 38 KJ/mol Tray efficiency is 50% Pressure in the condenser is 35 psia Pressure in the reboiler is 45 psia Surface tension in the column is 28.6 dyne/cm Weight of the column heads is 10% of the weight of the shell Thickness of column (shell) = 1/4 inches Material construction: Carbon steel Tray spacing: 24 inches Density of carbon steel= 7,833 Kg/m Distillation Column Pump Feed Storage Tank I Figure 1









