Answered step by step
Verified Expert Solution
Question
1 Approved Answer
determine constants A12 and A21 using following methods - VLE data for Ethanol (1) - Benzene (2) at 101.325 kPa are provided below. Xi yi
determine constants A12 and A21 using following methods 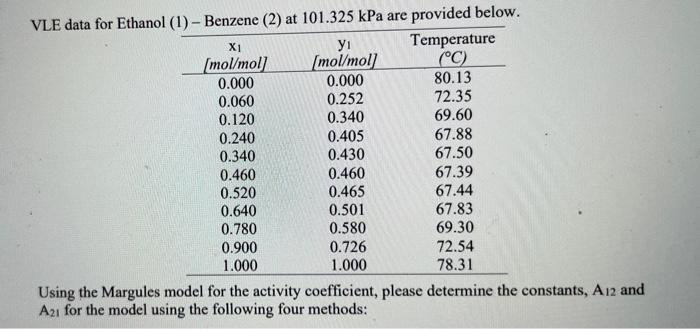

- VLE data for Ethanol (1) - Benzene (2) at 101.325 kPa are provided below. Xi yi Temperature (mol/mol] (mol/mol] (C) 0.000 0.000 80.13 0.060 0.252 72.35 0.120 0.340 69.60 0.240 0.405 67.88 0.340 0.430 67.50 0.460 0.460 67.39 0.520 0.465 67.44 0.640 0.501 67.83 0.780 0.580 69.30 0.900 0.726 72.54 1.000 1.000 78.31 Using the Margules model for the activity coefficient, please determine the constants, A12 and A2, for the model using the following four methods: (b) Linearizing the data as GF/x4x2RT); (c) Using only the azeotrope point (y1 = x1); and (d) Best overall fit based on the sum of square of the errors. - VLE data for Ethanol (1) - Benzene (2) at 101.325 kPa are provided below. Xi yi Temperature (mol/mol] (mol/mol] (C) 0.000 0.000 80.13 0.060 0.252 72.35 0.120 0.340 69.60 0.240 0.405 67.88 0.340 0.430 67.50 0.460 0.460 67.39 0.520 0.465 67.44 0.640 0.501 67.83 0.780 0.580 69.30 0.900 0.726 72.54 1.000 1.000 78.31 Using the Margules model for the activity coefficient, please determine the constants, A12 and A2, for the model using the following four methods: (b) Linearizing the data as GF/x4x2RT); (c) Using only the azeotrope point (y1 = x1); and (d) Best overall fit based on the sum of square of the errors 

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


