Question
Give the exact parent peak mass of the compound C6H6C16. Assume that ONLY the most common isotope is present. Give the mass to 4
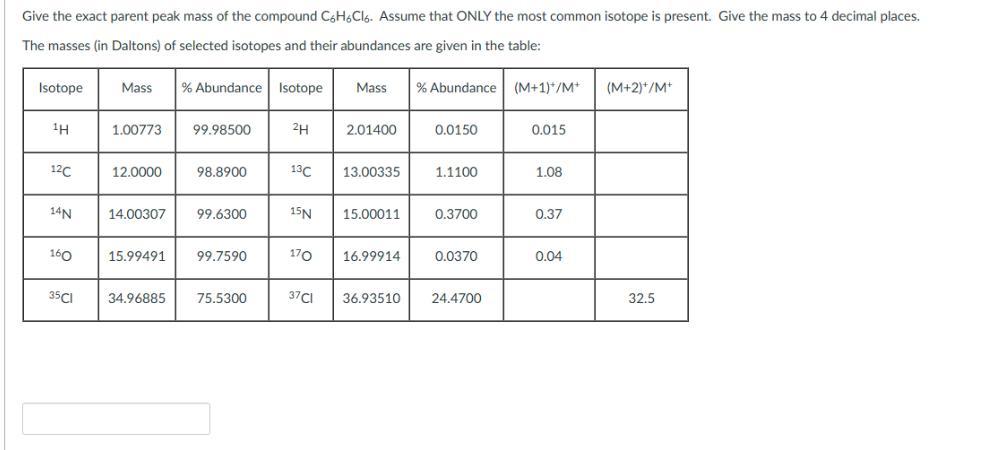
Give the exact parent peak mass of the compound C6H6C16. Assume that ONLY the most common isotope is present. Give the mass to 4 decimal places. The masses (in Daltons) of selected isotopes and their abundances are given in the table: Isotope Mass % Abundance Isotope Mass % Abundance (M+1)*/M+ (M+2)+/M+ 1H 1.00773 99.98500 2H 2.01400 0.0150 0.015 12C 12.0000 98.8900 13C 13.00335 1.1100 1.08 14N 14.00307 99.6300 15N 15.00011 0.3700 0.37 160 15.99491 99.7590 170 16.99914 0.0370 0.04 35CI 34.96885 75.5300 37CI 36.93510 24.4700 32.5
Step by Step Solution
There are 3 Steps involved in it
Step: 1
To find the parent peak mass of the compound C6H6Cl6 we need to ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Essentials Of Corporate Finance
Authors: Stephen Ross, Randolph Westerfield, Bradford Jordan
7th Edition
0073382469, 978-0073382463
Students also viewed these Finance questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



