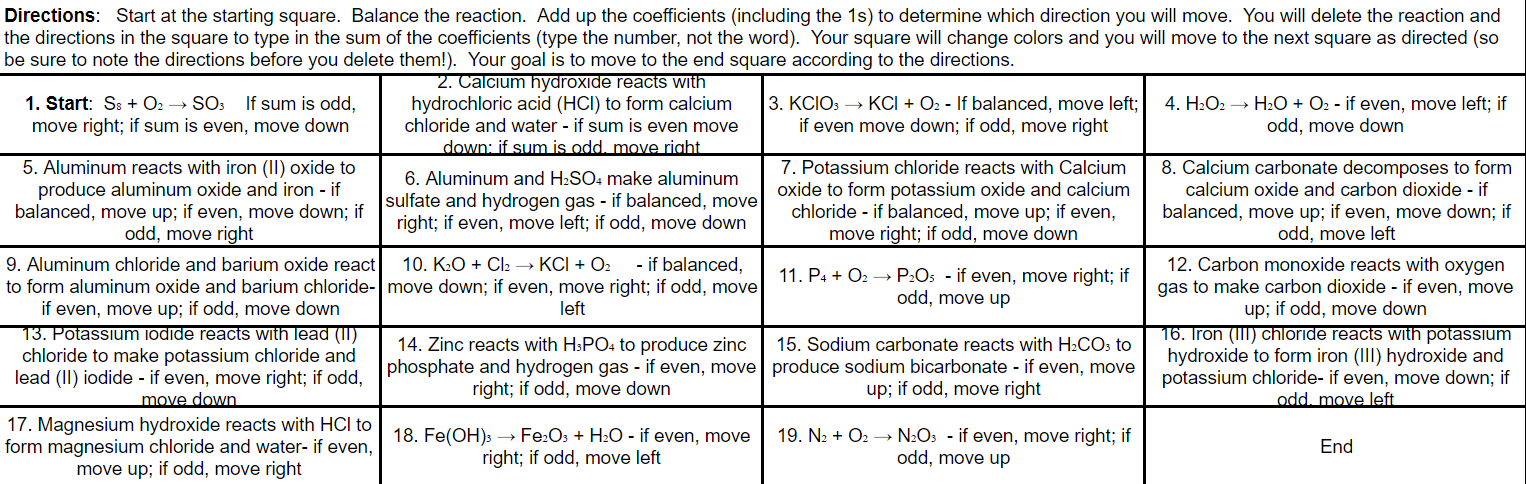
Directions: Start at the starting square. Balance the reaction. Add up the coefficients (including the 1s) to determine which direction you will move. You will delete the reaction and the directions in the square to type in the sum of the coefficients (type the number, not the word). Your square will change colors and you will move to the next square as directed (so be sure to note the directions before you delete them!). Your goal is to move to the end square according to the directions. 2. Calcium hydroxide reacts with 1. Start: S: + O2 SO3 If sum is odd, hydrochloric acid (HCI) to form calcium 3. KCIO: KCI + O2 - If balanced, move left; 4. H202 + H2O + O2 - if even, move left; if move right; if sum is even, move down chloride and water - if sum is even move if even move down; if odd, move right odd, move down down: if sum is odd. move right 5. Aluminum reacts with iron (11) oxide to 7. Potassium chloride reacts with Calcium 8. Calcium carbonate decomposes to form 6. Aluminum and H2SO4 make aluminum produce aluminum oxide and iron - if oxide to form potassium oxide and calcium calcium oxide and carbon dioxide - if balanced, move up; if even, move down: if sulfate and hydrogen gas - if balanced, move chloride - if balanced, move up; if even, balanced, move up; if even, move down; if odd, move right right; if even, move left; if odd, move down move right; if odd, move down odd, move left 9. Aluminum chloride and barium oxide react 10. K2O + Cl2 KCI + O2 - if balanced, 11. P4 + O2 P2O5 - if even, move right; if 12. Carbon monoxide reacts with oxygen to form aluminum oxide and barium chloride-move down; if even, move right; if odd, move odd, move up gas to make carbon dioxide - if even, move if even, move up; if odd, move down left up; if odd, move down 13. Potassium iodide reacts with lead (11) 16. Iron (III) chloride reacts with potassium 14. Zinc reacts with H3PO4 to produce zinc 15. Sodium carbonate reacts with H2CO3 to chloride to make potassium chloride and lead (II) iodide - if even, move right; if odd, phosphate and hydrogen gas - if even, move produce sodium bicarbonate - if even, move hydroxide to form iron (III) hydroxide and right; if odd, move down up; if odd, move right potassium chloride- if even, move down; if move down odd. move left 17. Magnesium hydroxide reacts with HCl to 18. Fe(OH)3 Fe2O3 + H2O - if even, move 19. N2 + O2N2O3 - if even, move right; if form magnesium chloride and water- if even, End right; if odd, move left odd, move up move up; if odd, move right







