Question
Q3. Calculate the percent ionization of Ibuprofen (a weak acid) in the stomach, at a pH of 3. The pK, of Ibuprofen is around

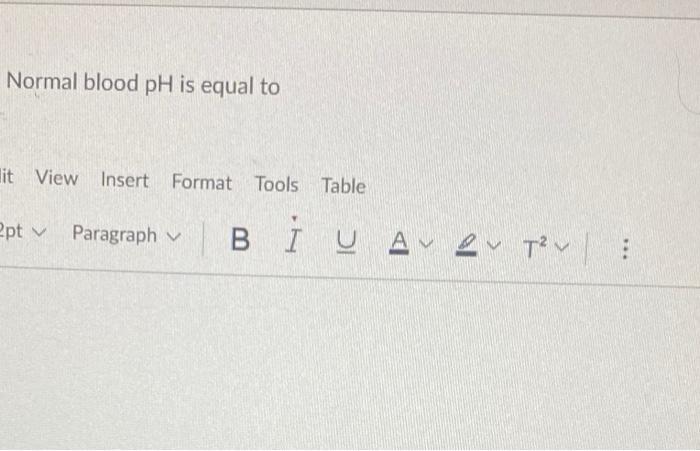
Q3. Calculate the percent ionization of Ibuprofen (a weak acid) in the stomach, at a pH of 3. The pK, of Ibuprofen is around 4.4. Normal blood pH is equal to it View Insert Format Tools Table 2pt v Paragraph V BIUA 2 TV :
Step by Step Solution
3.56 Rating (156 Votes )
There are 3 Steps involved in it
Step: 1
the normal pH range is 735 745 P...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Economics
Authors: Roger A. Arnold
12th edition
978-1305758674, 1305758676, 978-1285738321
Students also viewed these General Management questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



