Answered step by step
Verified Expert Solution
Question
1 Approved Answer
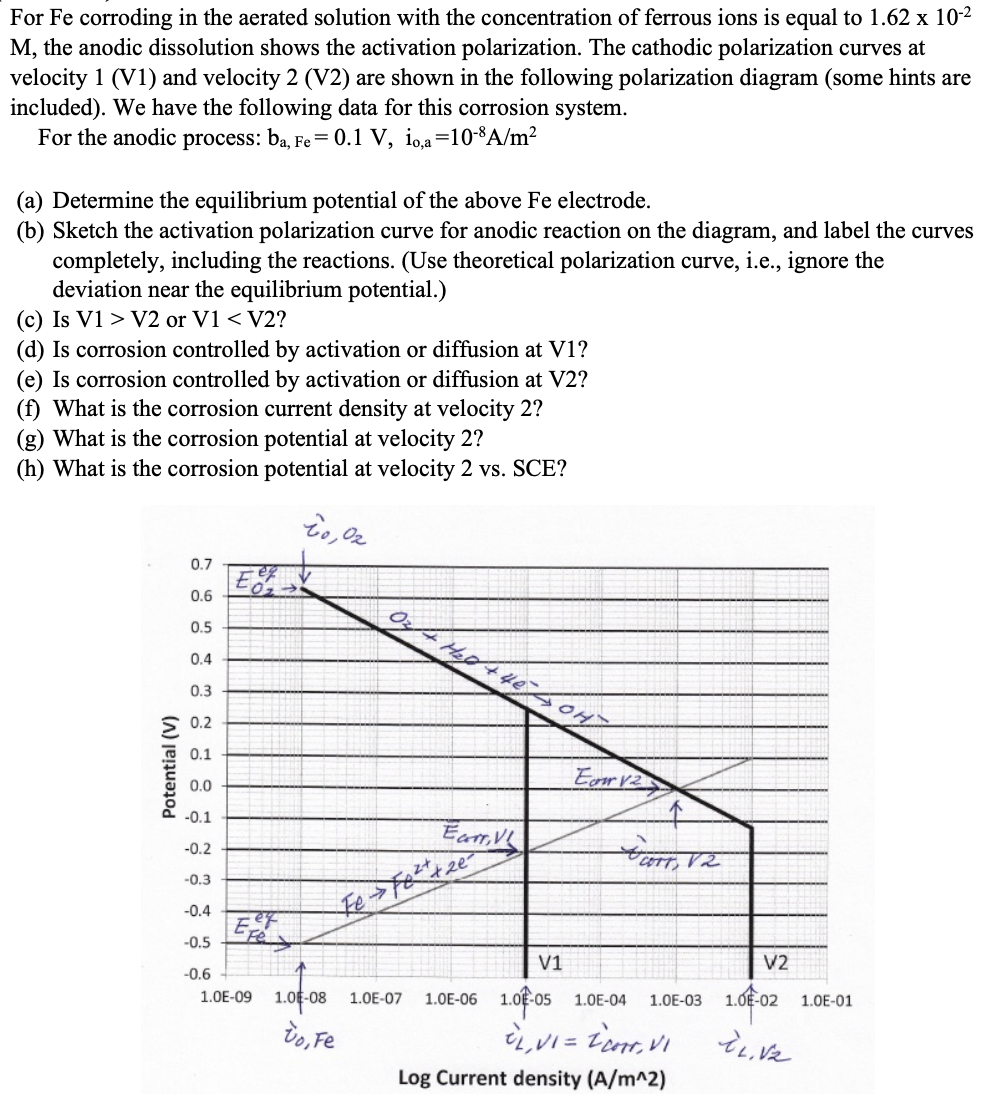
Do For Fe corroding in the aerated solution with the concentration of ferrous ions is equal to 1 . 6 2 1 0 - 2
Do For Fe corroding in the aerated solution with the concentration of ferrous ions is equal to
the anodic dissolution shows the activation polarization. The cathodic polarization curves at
velocity V and velocity V are shown in the following polarization diagram some hints are
included We have the following data for this corrosion system.
For the anodic process:
a Determine the equilibrium potential of the above Fe electrode.
b Sketch the activation polarization curve for anodic reaction on the diagram, and label the curves
completely, including the reactions. Use theoretical polarization curve, ie ignore the
deviation near the equilibrium potential.
c Is or
corrosion controlled activation diffusion
corrosion controlled activation diffusion
What the corrosion current density velocity
What the corrosion potential velocity
What the corrosion potential velocity SCE?
Do not use ChatGPT or other chegg answers.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


