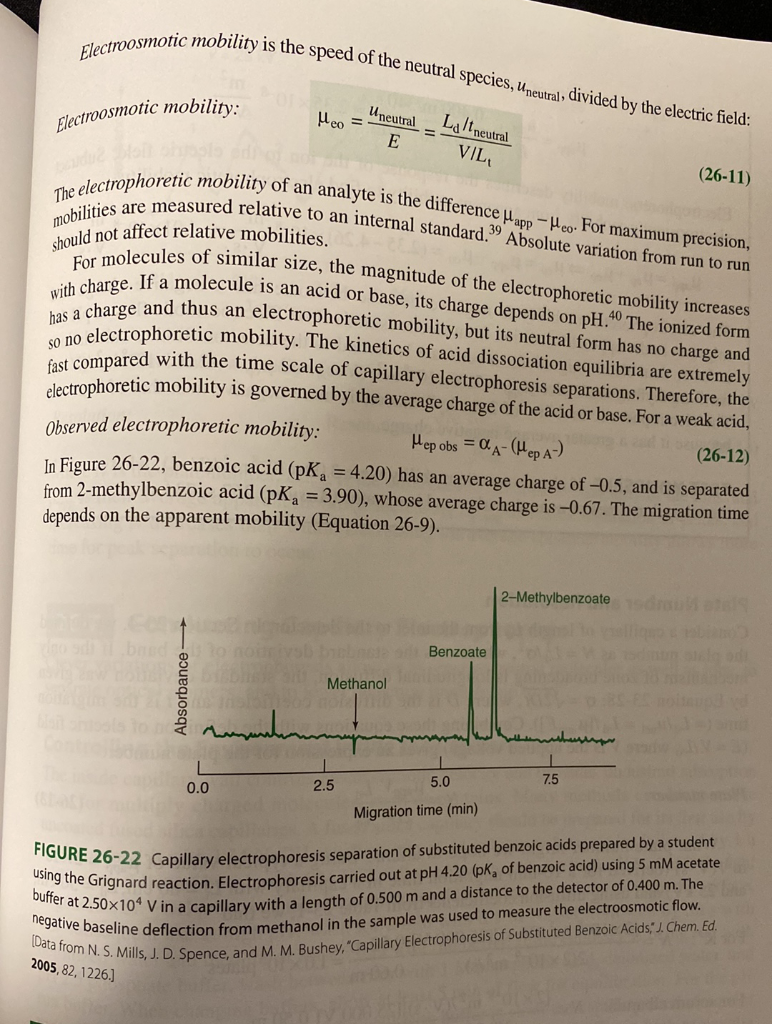

Electroosmotic mobility is the speed of the neutral species, Uneutral, divided by the electric field: Electroosmotic mobility: Uneutral E Lj/t neutral VIL should not affect relative mobilities. Meo (26-11) The electrophoretic mobility of an analyte is the difference Mapp - Heo For maximum precision, mobilities are measured relative to an internal standard. 19 Absolute variation from run to run For molecules of similar size, the magnitude of the electrophoretic mobility increases with charge. If a molecule is an acid or base, its charge depends on pH.40 The ionized form has a charge and thus an electrophoretic mobility, but its neutral form has no charge and so no electrophoretic mobility. The kinetics of acid dissociation equilibria are extremely fast compared with the time scale of capillary electrophoresis separations. Therefore, the electrophoretic mobility is governed by the average charge of the acid or base. For a weak acid, Observed electrophoretic mobility: Mep obs = Q A-(Mep A) (26-12) In Figure 26-22, benzoic acid (pK= 4.20) has an average charge of -0.5, and is separated from 2-methylbenzoic acid (pKg = 3.90), whose average charge is -0.67. The migration time depends on the apparent mobility (Equation 26-9). 2-Methylbenzoate Benzoate Methanol to mahan 1 2.5 7.5 0.0 5.0 Migration time (min) FIGURE 26-22 Capillary electrophoresis separation of substituted benzoic acids prepared by a student using the Grignard reaction. Electrophoresis carried out at pH 4.20 (pk, of benzoic acid) using 5 mM acetate buffer at 2.50X104 V in a capillary with a length of 0.500 m and a distance to the detector of 0.400 m. The negative baseline deflection from methanol in the sample was used to measure the electroosmotic flow. (Data from N. S. Mills, J. D. Spence, and M. M. Bushey, "Capillary Electrophoresis of Substituted Benzoic Acids," J. Chem. Ed. 2005, 82,1226. 26-31. Figure 26-22 shows the separation of substituted benzoates. There is a peak of unknown identity at 86.0 s. (a) Is the unknown a cation, neutral, or an anion? (b) Find the apparent mobility and electrophoretic mobility of the unknown peak. Electroosmotic mobility is the speed of the neutral species, Uneutral, divided by the electric field: Electroosmotic mobility: Uneutral E Lj/t neutral VIL should not affect relative mobilities. Meo (26-11) The electrophoretic mobility of an analyte is the difference Mapp - Heo For maximum precision, mobilities are measured relative to an internal standard. 19 Absolute variation from run to run For molecules of similar size, the magnitude of the electrophoretic mobility increases with charge. If a molecule is an acid or base, its charge depends on pH.40 The ionized form has a charge and thus an electrophoretic mobility, but its neutral form has no charge and so no electrophoretic mobility. The kinetics of acid dissociation equilibria are extremely fast compared with the time scale of capillary electrophoresis separations. Therefore, the electrophoretic mobility is governed by the average charge of the acid or base. For a weak acid, Observed electrophoretic mobility: Mep obs = Q A-(Mep A) (26-12) In Figure 26-22, benzoic acid (pK= 4.20) has an average charge of -0.5, and is separated from 2-methylbenzoic acid (pKg = 3.90), whose average charge is -0.67. The migration time depends on the apparent mobility (Equation 26-9). 2-Methylbenzoate Benzoate Methanol to mahan 1 2.5 7.5 0.0 5.0 Migration time (min) FIGURE 26-22 Capillary electrophoresis separation of substituted benzoic acids prepared by a student using the Grignard reaction. Electrophoresis carried out at pH 4.20 (pk, of benzoic acid) using 5 mM acetate buffer at 2.50X104 V in a capillary with a length of 0.500 m and a distance to the detector of 0.400 m. The negative baseline deflection from methanol in the sample was used to measure the electroosmotic flow. (Data from N. S. Mills, J. D. Spence, and M. M. Bushey, "Capillary Electrophoresis of Substituted Benzoic Acids," J. Chem. Ed. 2005, 82,1226. 26-31. Figure 26-22 shows the separation of substituted benzoates. There is a peak of unknown identity at 86.0 s. (a) Is the unknown a cation, neutral, or an anion? (b) Find the apparent mobility and electrophoretic mobility of the unknown peak








