Question
ePredict the equilibrium concentration of CO_(2) in the reaction described below (for which Kc=5.1 at a temperature of 700K ) by constructing an equilibrium expression
ePredict the equilibrium concentration of
CO_(2)in the reaction described\ below (for which
Kc=5.1at a temperature of
700K) by constructing an\ equilibrium expression for Qc, constructing an ICE table, writing an\ equilibrium expression for
Kc, and solving for the equilibrium\ concentration. Complete Parts 1-4 before submitting your answer.\
CO(g)+H_(2)O(g)CO_(2)(g)+H_(2)(g)\ 1\ In a
2.0Lcontainer at
700K,0.13molCO,0.56molH_(2)O,1.62molCO_(2), and
0.54molH_(2)\ are allowed to react. Set up the expression for Qc. Each reaction participant must be\ represented by one tile. Do not combine terms.\ Once the expression is constructed, solve for Qc to determine the direction of the\ reaction.\
Q_(c)== 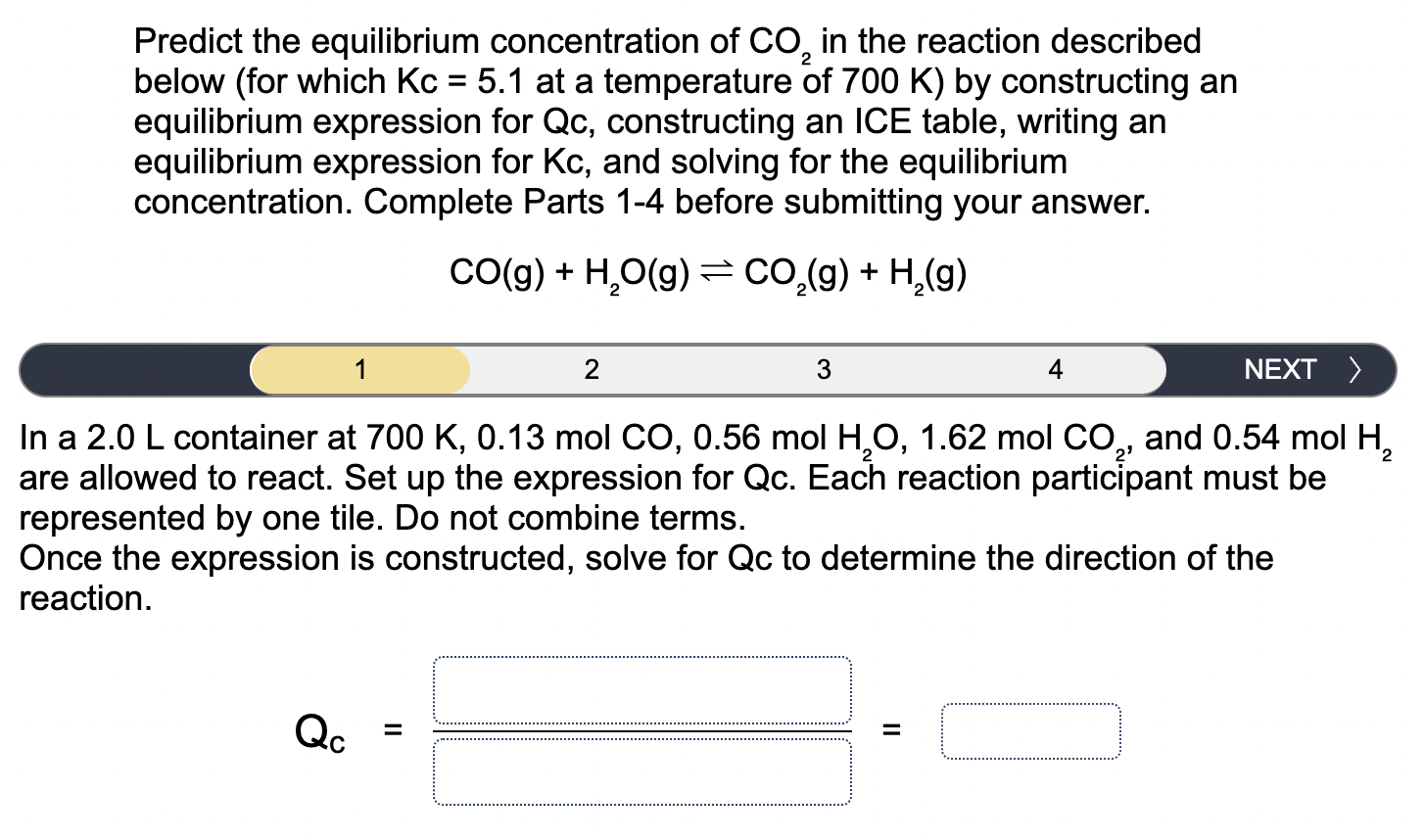
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


