Question
Estimate the equilibrium constant, at 500 and at 1000 K considering the full effect of temperature, using the graph ln K vs 1/T; compare with
Estimate the equilibrium constant, at 500 and at 1000 K considering the full effect of temperature, using the graph ln K vs 1/T; compare with the results of the previous point and analyze.
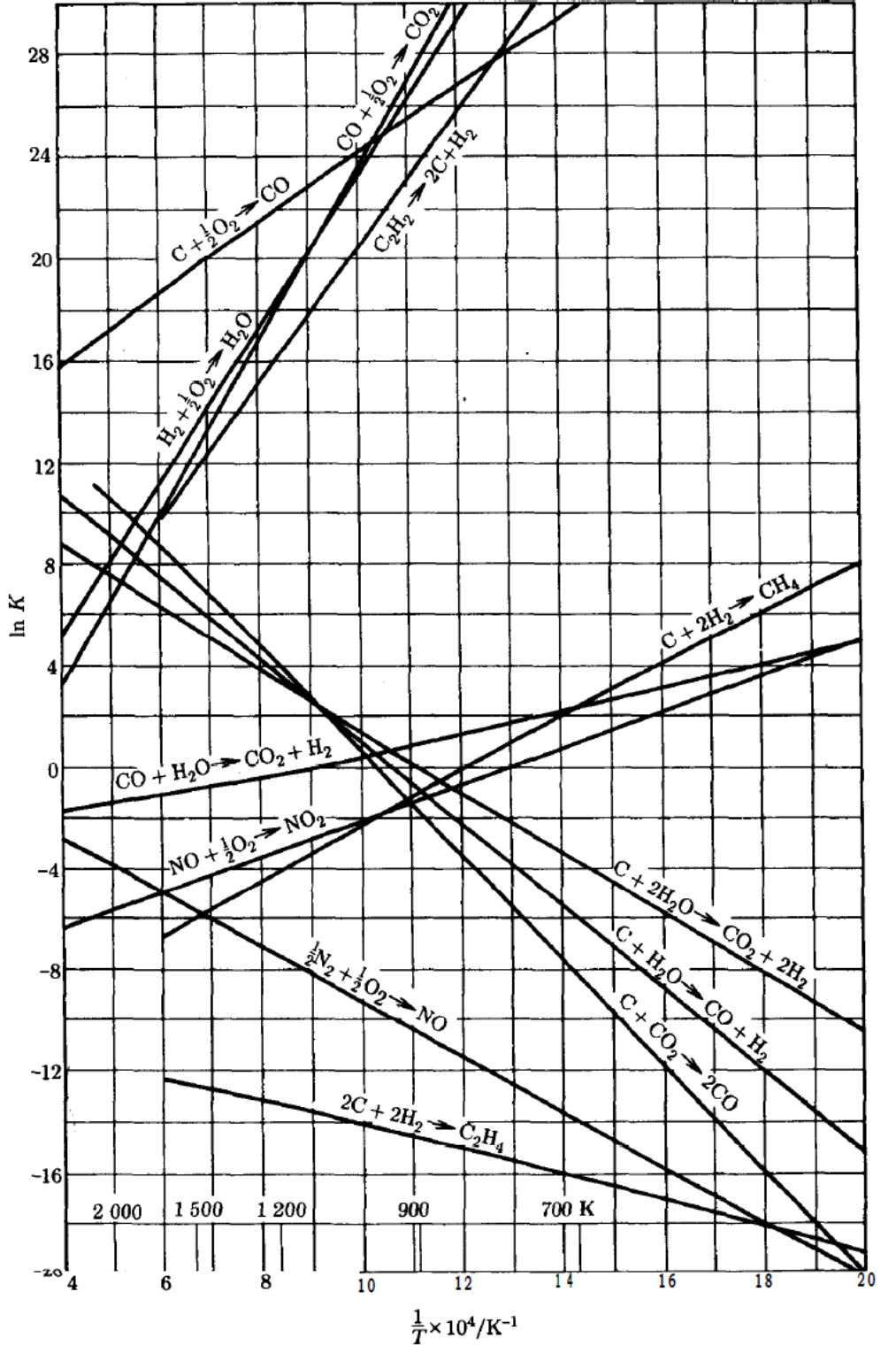
If the process is carried out in an adiabatic catalytic reactor where pure ethylene is fed at 1530 K and 1 bar, considering that the mixture behaves like an ideal gas, calculate the conditions of the mixture obtained. (This point is the most important)
If the calculation requires an iterative process, you must perform an iteration and explain the procedure.
Perform the iterative calculation
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


