Question
Ethyl alcohol (C2H5OH) is to be recovered from an aqueous solution by continuous distillation. The feed will contain 10% w/w ethyl alcohol. Ethyl alcohol of
Ethyl alcohol (C2H5OH) is to be recovered from an aqueous solution by continuous distillation. The feed will contain 10% w/w ethyl alcohol. Ethyl alcohol of at least 75% purity is required(top product), and the bottom product must not contain more than 0.9 % alcohol. The optimum reflux ratio is 1.84R min. The number of plates equivalent to one theoretical plate is 1.45. Amount of steam of C 2H 5OH in the alcohol-water mixture is 1m 3/s (at point 4 of the diagram). Pressure in the column is 1 atm. Design a distillation column, reboiler and condenser suitable for conducting the process. Water with the temperature of 25C is available for cooling condenser. Temperature of the feed is 23C. Steam at 2.54 atm pressure is used for heating the column. The overall heat transfer coefficient of the reboiler is 2500W/m 2 C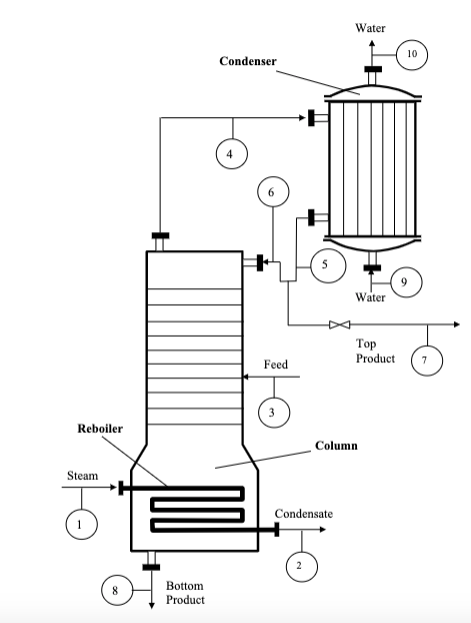
Boiling point (1atm, 100% alcohol) - 78.3C Density (100% alcohol, 25C) - 789kg/m 3 Specific heat (100% alcohol, 25C) - 0.61 kcal / kgC Latent heat of vaporisation (100% alcohol, 78.3C) - 834.3 kJ/kg Enthalpy of water steam (2.54 atm, 128C) - 2718 kJ/kg Enthalpy of water (2.54 atm, 128C) - 538 kJ/kg
Complete material balance and energy balance calculations for each point on the figure.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


