Answered step by step
Verified Expert Solution
Question
1 Approved Answer
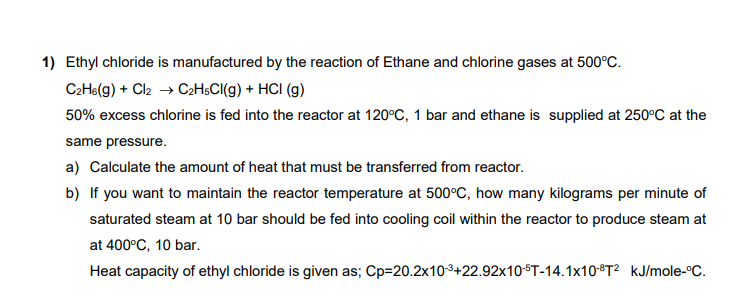
Ethyl chloride is manufactured by the reaction of Ethane and chlorine gases at 5 0 0 C . C 2 H 6 ( g )
Ethyl chloride is manufactured by the reaction of Ethane and chlorine gases at
excess chlorine is fed into the reactor at bar and ethane is supplied at at the
same pressure.
a Calculate the amount of heat that must be transferred from reactor.
b If you want to maintain the reactor temperature at how many kilograms per minute of
saturated steam at bar should be fed into cooling coil within the reactor to produce steam at
at bar.
Heat capacity of ethyl chloride is given as; ole
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


