Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Ethylene oxide (C2H4O) is an intermediate compound that is produced in large quantities to be used in the production of glycol and polyethylene glycol. Ethylene
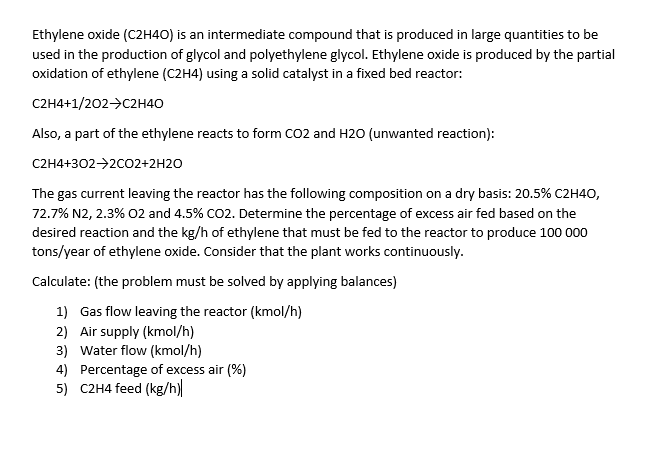 Ethylene oxide (C2H4O) is an intermediate compound that is produced in large quantities to be used in the production of glycol and polyethylene glycol. Ethylene oxide is produced by the partial oxidation of ethylene (C2H4) using a solid catalyst in a fixed bed reactor: C2H4+1/2O2C2H4O Also, a part of the ethylene reacts to form CO2 and H2O (unwanted reaction): C2H4+3O22CO2+2H2O The gas current leaving the reactor has the following composition on a dry basis: 20.5%C2H4O, 72.7%N2,2.3%O2 and 4.5%CO2. Determine the percentage of excess air fed based on the desired reaction and the kg/h of ethylene that must be fed to the reactor to produce 100000 tons/year of ethylene oxide. Consider that the plant works continuously. Calculate: (the problem must be solved by applying balances) 1) Gas flow leaving the reactor (kmol/h) 2) Air supply (kmol/h) 3) Water flow (kmol/h) 4) Percentage of excess air (\%) 5) C2H4 feed (kg/h) Ethylene oxide (C2H4O) is an intermediate compound that is produced in large quantities to be used in the production of glycol and polyethylene glycol. Ethylene oxide is produced by the partial oxidation of ethylene (C2H4) using a solid catalyst in a fixed bed reactor: C2H4+1/2O2C2H4O Also, a part of the ethylene reacts to form CO2 and H2O (unwanted reaction): C2H4+3O22CO2+2H2O The gas current leaving the reactor has the following composition on a dry basis: 20.5%C2H4O, 72.7%N2,2.3%O2 and 4.5%CO2. Determine the percentage of excess air fed based on the desired reaction and the kg/h of ethylene that must be fed to the reactor to produce 100000 tons/year of ethylene oxide. Consider that the plant works continuously. Calculate: (the problem must be solved by applying balances) 1) Gas flow leaving the reactor (kmol/h) 2) Air supply (kmol/h) 3) Water flow (kmol/h) 4) Percentage of excess air (\%) 5) C2H4 feed (kg/h)
Ethylene oxide (C2H4O) is an intermediate compound that is produced in large quantities to be used in the production of glycol and polyethylene glycol. Ethylene oxide is produced by the partial oxidation of ethylene (C2H4) using a solid catalyst in a fixed bed reactor: C2H4+1/2O2C2H4O Also, a part of the ethylene reacts to form CO2 and H2O (unwanted reaction): C2H4+3O22CO2+2H2O The gas current leaving the reactor has the following composition on a dry basis: 20.5%C2H4O, 72.7%N2,2.3%O2 and 4.5%CO2. Determine the percentage of excess air fed based on the desired reaction and the kg/h of ethylene that must be fed to the reactor to produce 100000 tons/year of ethylene oxide. Consider that the plant works continuously. Calculate: (the problem must be solved by applying balances) 1) Gas flow leaving the reactor (kmol/h) 2) Air supply (kmol/h) 3) Water flow (kmol/h) 4) Percentage of excess air (\%) 5) C2H4 feed (kg/h) Ethylene oxide (C2H4O) is an intermediate compound that is produced in large quantities to be used in the production of glycol and polyethylene glycol. Ethylene oxide is produced by the partial oxidation of ethylene (C2H4) using a solid catalyst in a fixed bed reactor: C2H4+1/2O2C2H4O Also, a part of the ethylene reacts to form CO2 and H2O (unwanted reaction): C2H4+3O22CO2+2H2O The gas current leaving the reactor has the following composition on a dry basis: 20.5%C2H4O, 72.7%N2,2.3%O2 and 4.5%CO2. Determine the percentage of excess air fed based on the desired reaction and the kg/h of ethylene that must be fed to the reactor to produce 100000 tons/year of ethylene oxide. Consider that the plant works continuously. Calculate: (the problem must be solved by applying balances) 1) Gas flow leaving the reactor (kmol/h) 2) Air supply (kmol/h) 3) Water flow (kmol/h) 4) Percentage of excess air (\%) 5) C2H4 feed (kg/h) Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


