Question
[15 pts] Plot P/P* = xewx/RT x1ewx/RT versus x for RT/w = 0.60, 0.50, 0.45, 0.40, and 0.35. Note that some of the curves
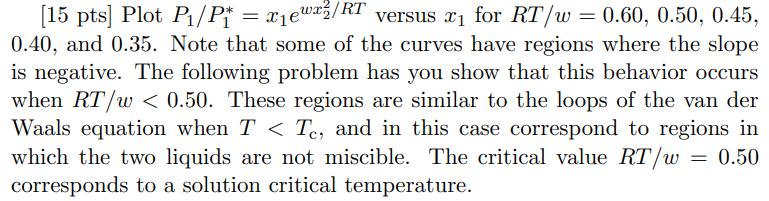
[15 pts] Plot P/P* = xewx/RT x1ewx/RT versus x for RT/w = 0.60, 0.50, 0.45, 0.40, and 0.35. Note that some of the curves have regions where the slope is negative. The following problem has you show that this behavior occurs when RT/w < 0.50. These regions are similar to the loops of the van der Waals equation when T < Te, and in this case correspond to regions in which the two liquids are not miscible. The critical value RT/w: = 0.50 corresponds to a solution critical temperature.
Step by Step Solution
3.38 Rating (160 Votes )
There are 3 Steps involved in it
Step: 1
For the given values of RTw the calculated values of P1P1 is given below in the table PP RT ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Ethics Theory and Contemporary Issues
Authors: Barbara MacKinnon, Andrew Fiala
8th edition
9781305162846, 1285196759, 1305162846, 978-1285196756
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



