Answered step by step
Verified Expert Solution
Question
1 Approved Answer
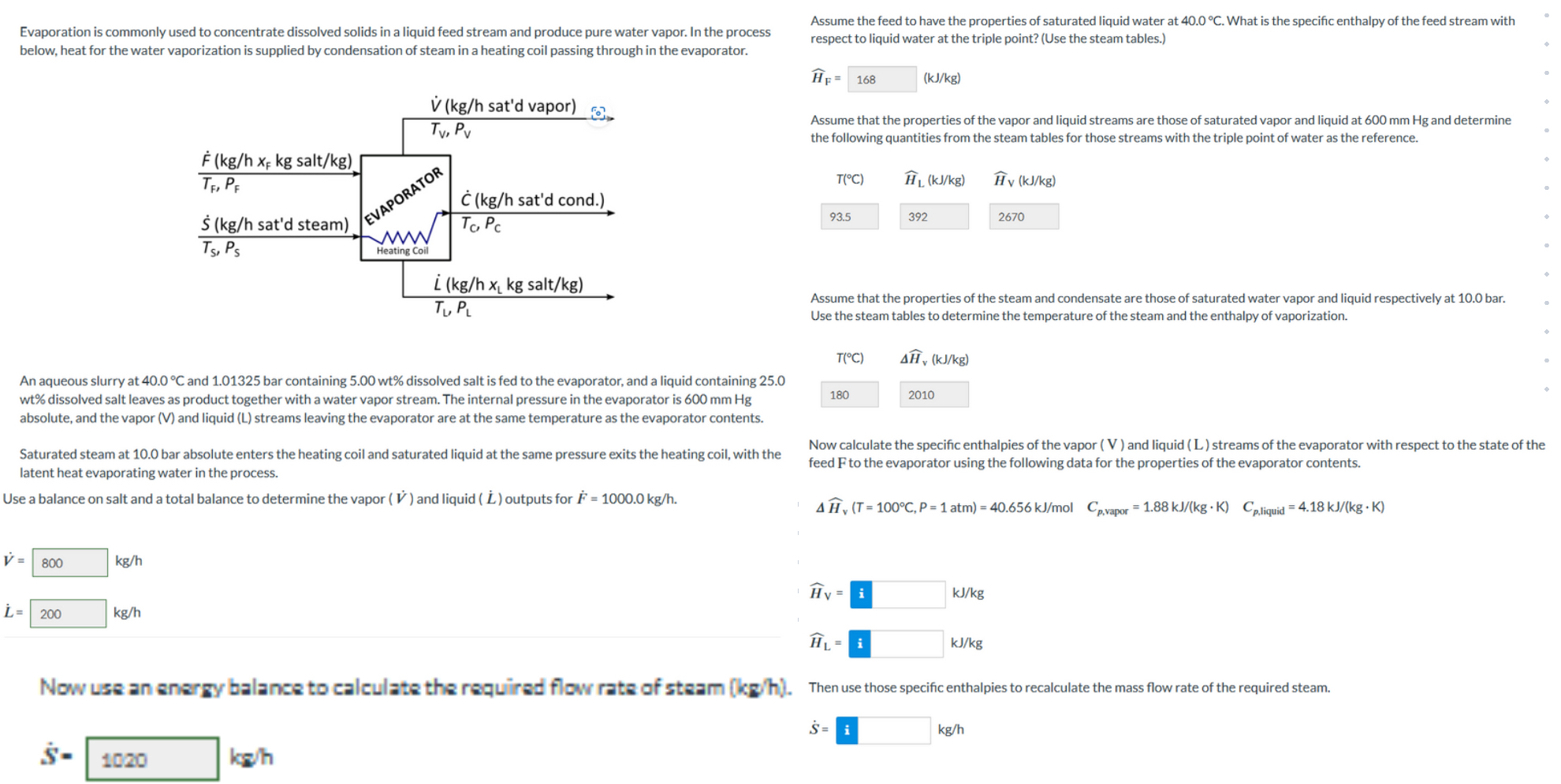
Evaporation is commonly used to concentrate dissolved solids in a liquid feed stream and produce pure water vapor. In the process below, heat for the
Evaporation is commonly used to concentrate dissolved solids in a liquid feed stream and produce pure water vapor. In the process below, heat for the water vaporization is supplied by condensation of steam in a heating coil passing through in the evaporator.
An aqueous slurry at and bar containing dissolved salt is fed to the evaporator, and a liquid containing wt dissolved salt leaves as product together with a water vapor stream. The internal pressure in the evaporator is absolute and the vapor and liquid streams leaving the evaporator are at the same temperature as the evaporator contents.
Saturated steam at bar absolute enters the heating coil and saturated liquid at the same pressure exits the heating coil, with the latent heat evaporating water in the process.
Use a balance on salt and a total balance to determine the vapor and liquid outputs for
Assume the feed to have the properties of saturated liquid water at What is the specific enthalpy of the feed stream with respect to liquid water at the triple point? Use the steam tables.
widehat
Assume that the properties of the vapor and liquid streams are those of saturated vapor and liquid at and determine the following quantities from the steam tables for those streams with the triple point of water as the reference.
widehatwidehat
Assume that the properties of the steam and condensate are those of saturated water vapor and liquid respectively at bar. Use the steam tables to determine the temperature of the steam and the enthalpy of vaporization.
Now calculate the specific enthalpies of the vapor V and liquid streams of the evaporator with respect to the state of the feed to the evaporator using the following data for the properties of the evaporator contents. Please help find the enthalpies of vapor and liquid along with the mass flow rate
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


