Answered step by step
Verified Expert Solution
Question
1 Approved Answer
EXAMPLE 12.11-2. Heat Balance in Crystallization A feed solution of 2268 kg at 327.6 K (54.4C) containing 48.2 kg MgSO./100 kg total water is cooled
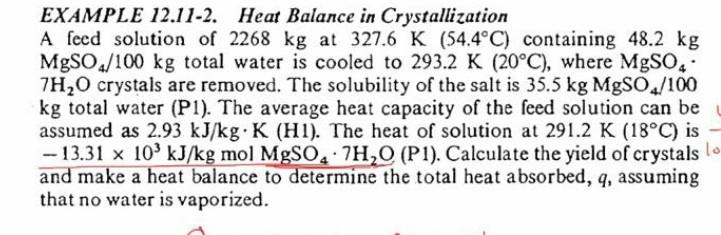
EXAMPLE 12.11-2. Heat Balance in Crystallization A feed solution of 2268 kg at 327.6 K (54.4C) containing 48.2 kg MgSO./100 kg total water is cooled to 293.2 K (20C), where MgSO. 7H2O crystals are removed. The solubility of the salt is 35.5 kg MgSO/100 kg total water (P1). The average heat capacity of the feed solution can be assumed as 2.93 kJ/kg.K (HI). The heat of solution at 291.2 K (18C) is -- 13.31 x 10 kJ/kg mol MgSO.7H2O (P1). Calculate the yield of crystals lo and make a heat balance to determine the total heat absorbed, q, assuming that no water is vaporized
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


