Answered step by step
Verified Expert Solution
Question
1 Approved Answer
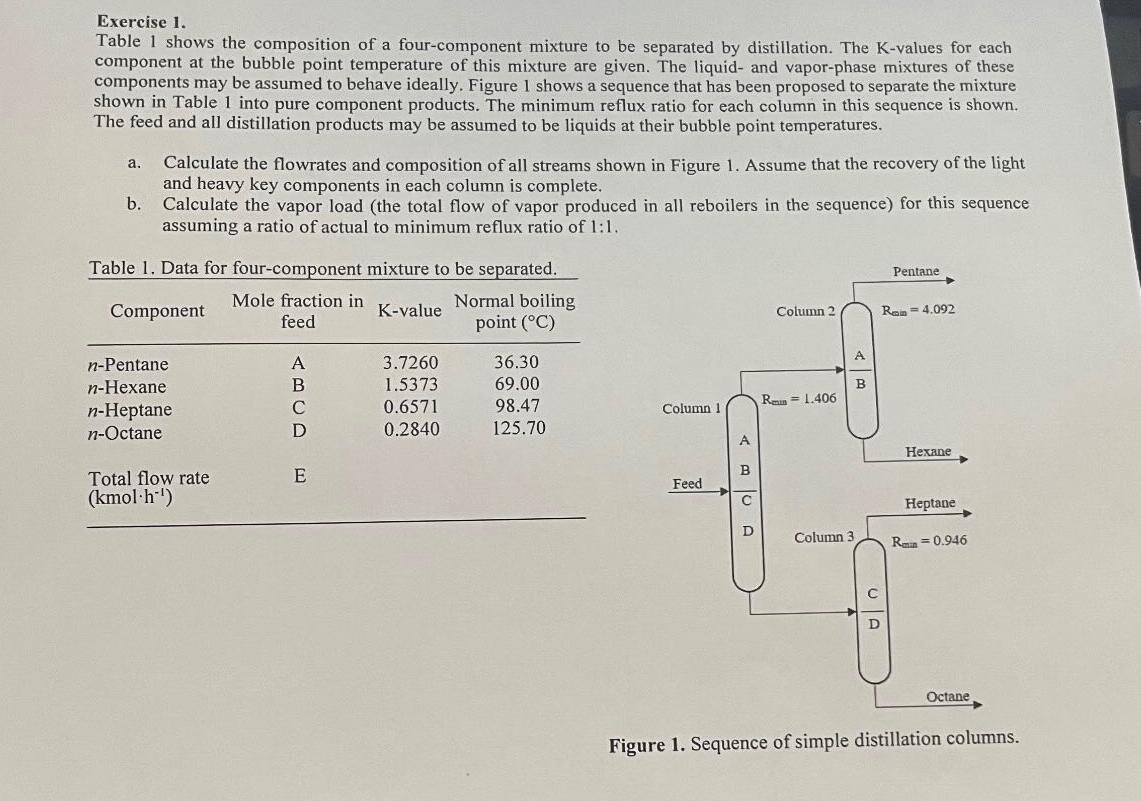
Exercise 1 . Table 1 shows the composition of a four - component mixture to be separated by distillation. The K - values for each
Exercise
Table shows the composition of a fourcomponent mixture to be separated by distillation. The Kvalues for each component at the bubble point temperature of this mixture are given. The liquid and vaporphase mixtures of these components may be assumed to behave ideally. Figure shows a sequence that has been proposed to separate the mixture shown in Table into pure component products. The minimum reflux ratio for each column in this sequence is shown. The feed and all distillation products may be assumed to be liquids at their bubble point temperatures.
a Calculate the flowrates and composition of all streams shown in Figure Assume that the recovery of the light and heavy key components in each column is complete.
b Calculate the vapor load the total flow of vapor produced in all reboilers in the sequence for this sequence assuming a ratio of actual to minimum reflux ratio of :
Table Data for fourcomponent mixture to be separated.
tableComponenttableMole fraction infeedKvalue,tableNormal boilingpoint
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


