Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Exercise 2 An azeotropic mixture of ethanol and water, which contains 95.6 wt.% of ethanol, boils at 78.15C. The vapor pressures of pure substances at
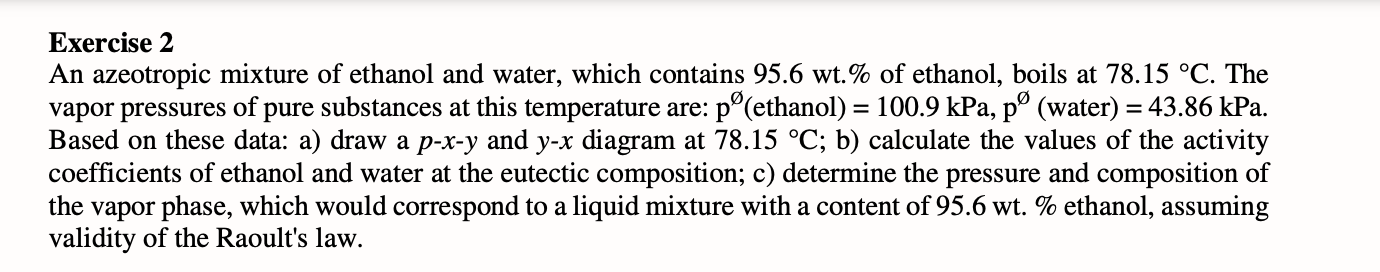 Exercise 2 An azeotropic mixture of ethanol and water, which contains 95.6 wt.\% of ethanol, boils at 78.15C. The vapor pressures of pure substances at this temperature are: p( ethanol) =100.9kPa,p (water) =43.86kPa. Based on these data: a) draw a pxy and y - x diagram at 78.15C; b) calculate the values of the activity coefficients of ethanol and water at the eutectic composition; c) determine the pressure and composition of the vapor phase, which would correspond to a liquid mixture with a content of 95.6wt% ethanol, assuming validity of the Raoult's law
Exercise 2 An azeotropic mixture of ethanol and water, which contains 95.6 wt.\% of ethanol, boils at 78.15C. The vapor pressures of pure substances at this temperature are: p( ethanol) =100.9kPa,p (water) =43.86kPa. Based on these data: a) draw a pxy and y - x diagram at 78.15C; b) calculate the values of the activity coefficients of ethanol and water at the eutectic composition; c) determine the pressure and composition of the vapor phase, which would correspond to a liquid mixture with a content of 95.6wt% ethanol, assuming validity of the Raoult's law Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


