Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Exercise #3: We wish to extract a mixture of 55wt% acetone and 45wt% water with methyl isobutyl ketone (MIK) at 298K and 1 bar. If
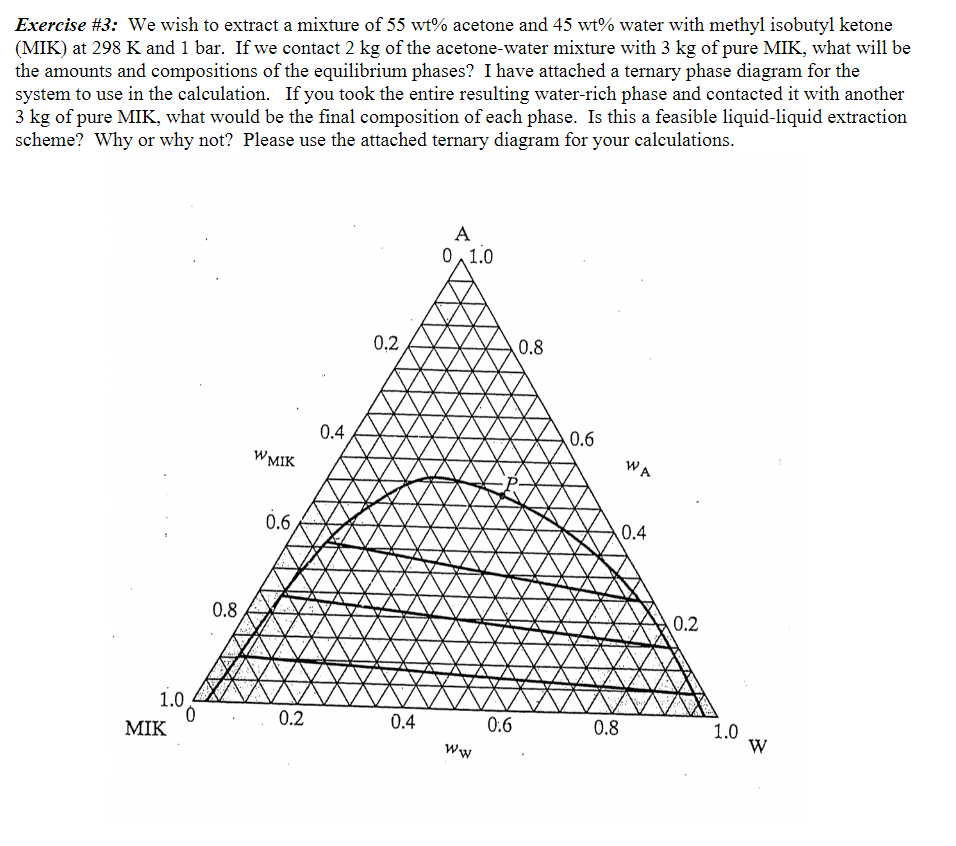 Exercise \#3: We wish to extract a mixture of 55wt% acetone and 45wt% water with methyl isobutyl ketone (MIK) at 298K and 1 bar. If we contact 2kg of the acetone-water mixture with 3kg of pure MIK, what will be the amounts and compositions of the equilibrium phases? I have attached a ternary phase diagram for the system to use in the calculation. If you took the entire resulting water-rich phase and contacted it with another 3kg of pure MIK, what would be the final composition of each phase. Is this a feasible liquid-liquid extraction scheme? Why or why not? Please use the attached ternary diagram for your calculations
Exercise \#3: We wish to extract a mixture of 55wt% acetone and 45wt% water with methyl isobutyl ketone (MIK) at 298K and 1 bar. If we contact 2kg of the acetone-water mixture with 3kg of pure MIK, what will be the amounts and compositions of the equilibrium phases? I have attached a ternary phase diagram for the system to use in the calculation. If you took the entire resulting water-rich phase and contacted it with another 3kg of pure MIK, what would be the final composition of each phase. Is this a feasible liquid-liquid extraction scheme? Why or why not? Please use the attached ternary diagram for your calculations Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


