Answered step by step
Verified Expert Solution
Question
1 Approved Answer
For Experiment 3 in Chem 255 Lab, students need to extract the fat trimyristin from the common cooking spice nutmeg before it can be
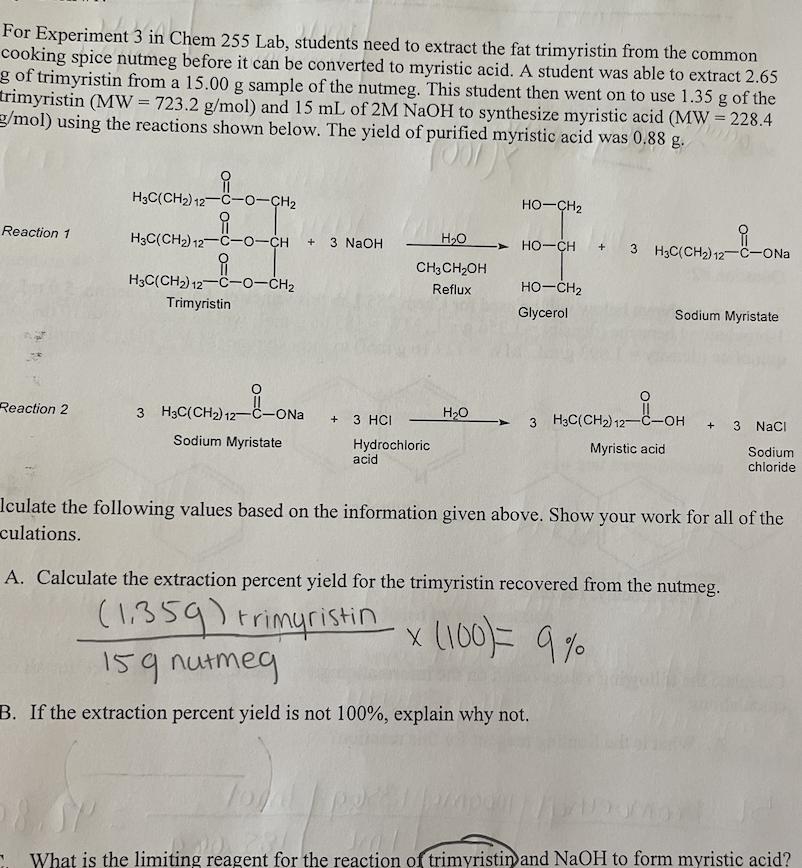
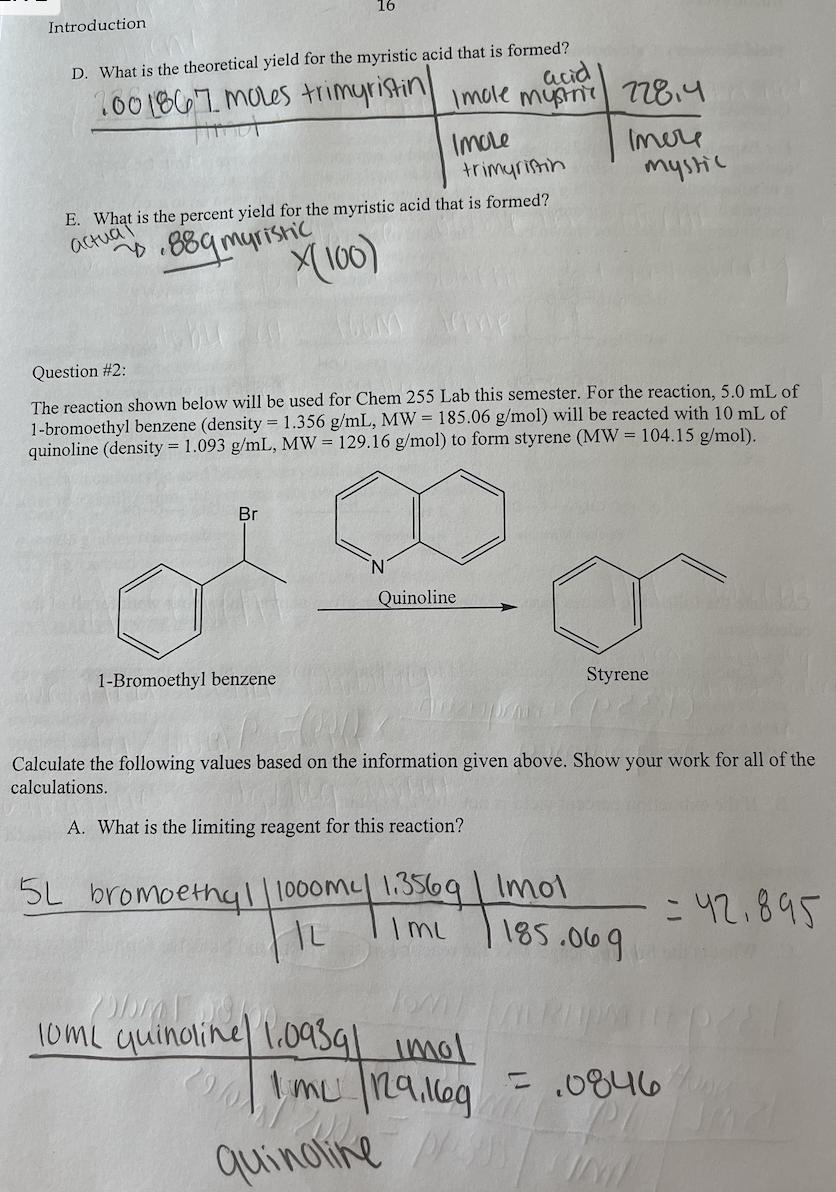

For Experiment 3 in Chem 255 Lab, students need to extract the fat trimyristin from the common cooking spice nutmeg before it can be converted to myristic acid. A student was able to extract 2.65 g of trimyristin from a 15.00 g sample of the nutmeg. This student then went on to use 1.35 g of the trimyristin (MW = 723.2 g/mol) and 15 mL of 2M NaOH to synthesize myristic acid (MW = 228.4 g/mol) using the reactions shown below. The yield of purified myristic acid was 0.88 g. Reaction 1 Reaction 2 H3C(CH2) 12-C-0-CH H3C(CH) 12-C-0-CH +3 NaOH H3C(CH) 12-C-0-CH Trimyristin 3 H3C(CH) 12- -ONa Sodium Myristate + 3 HCI HO CH3CHOH Reflux Hydrochloric acid HO HOCH2 HO-CH3 H3C(CH) 12-C-ONa Jawap HCH2 Glycerol __8_OH 3 H3C(CH2) 12- Sodium Myristate Myristic acid + 3 NaCl Iculate the following values based on the information given above. Show your work for all of the culations. A. Calculate the extraction percent yield for the trimyristin recovered from the nutmeg. (1359) trimyristinx (100) = 9% 159 nutmeg B. If the extraction percent yield is not 100%, explain why not. Sodium chloride What is the limiting reagent for the reaction of trimyristin and NaOH to form myristic acid? Introduction D. What is the theoretical yield for the myristic acid that is formed? 100 1867 moles trimyristin Hind acid Imole mystn 228.4 Imole trimyristin E. What is the percent yield for the myristic acid that is formed? actual 889 myristic X(100) 16 5L Question #2: The reaction shown below will be used for Chem 255 Lab this semester. For the reaction, 5.0 mL of 1-bromoethyl benzene (density = 1.356 g/mL, MW = 185.06 g/mol) will be reacted with 10 mL of quinoline (density= 1.093 g/mL, MW = 129.16 g/mol) to form styrene (MW = 104.15 g/mol). Br 1-Bromoethyl benzene Quinoline Imere mystic AZ Calculate the following values based on the information given above. Show your work for all of the calculations. A. What is the limiting reagent for this reaction? bromoethyl/1000my 1.3569 | Imol |L I mu 10mL quinoline 1.0939| imol 1 mu 129.169 quinoline // Styrene 185.069 = 42.895 = .0846/00g hil B. What is the theoretical yield for the reaction? 10/1/50846 mol quinaine Imole percent yield OH ole istiting quina he Strene n C. In Exp. 7, the styrene that is produced will be used to make polystyrene. In order to determine the success of the reaction, a ratio of the mass of polystyrene produced is compared to the mass of styrene used in the reaction. What type of calculation should be used for this comparison? 104,159 Imele Question #3: For Experiment 11 in Chem 255 lab, a student used 5.0 mL of 3- octanol (density = 0.818 g/mL, MW= 130.23 g/mol) and allowed it to react with excess HSO4 to form a variety of octenes (MW = 112.24 g/mol). The reaction is shown below. IML 71-8.81902 HSO4 cis- and trans-2-octene cis- and trans-3-octene Calculate the theoretical yield for the octenes produced by this reaction. SmL3-octanol.8189 | Imol | 112.249 130.239 Imol pub= 3,5250 Ormoctar 11 504
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


