Answered step by step
Verified Expert Solution
Question
1 Approved Answer
explain each step in detail please. Question 4: The product from the stripping section of a natural gas absorption plant has the following composition: Component
explain each step in detail please. 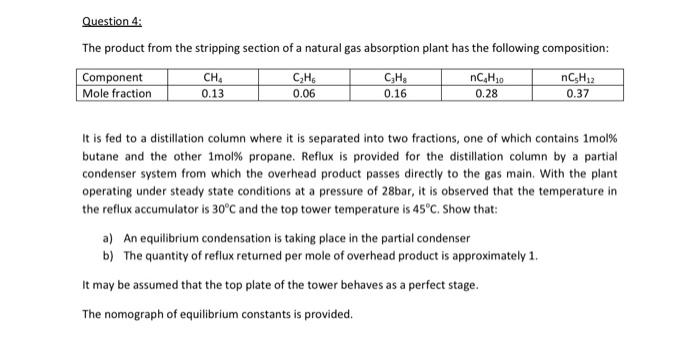
Question 4: The product from the stripping section of a natural gas absorption plant has the following composition: Component Mole fraction CH 0.13 CzHs 0.06 CHE 0.16 nC H30 0.28 nCH2 0.37 It is fed to a distillation column where it is separated into two fractions, one of which contains Imol% butane and the other Imol% propane. Reflux is provided for the distillation column by a partial condenser system from which the overhead product passes directly to the gas main. With the plant operating under steady state conditions at a pressure of 28bar, it is observed that the temperature in the reflux accumulator is 30C and the top tower temperature is 45C. Show that: a) An equilibrium condensation is taking place in the partial condenser b) The quantity of reflux returned per mole of overhead product is approximately 1. It may be assumed that the top plate of the tower behaves as a perfect stage. The nomograph of equilibrium constants is provided 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


