Answered step by step
Verified Expert Solution
Question
1 Approved Answer
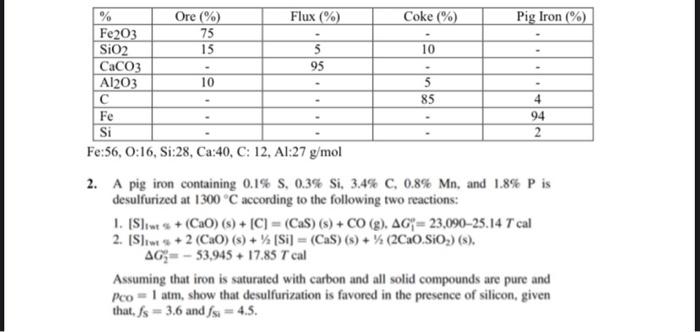
Fe:56,O:16,Si:28,Ca:40,C:12,Al:27g/mol 2. A pig iron containing 0.1%S,0.3%Si,3.4%C,0.8%Mn, and 1.8%P is desulfurized at 1300C according to the following two reactions: 1. [S]iwts+(CaO)(s)+[C]=(CaS)(s)+CO(g),G1e=23,09025.14Tcal 2. [S]iws+2(CaO)(s)+1/2[Si]=(CaS)(s)+1/2(2CaO.SiO2)(s). G2=53,945+17.85Tcal Assuming
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


