Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Few researchers studied the kinetics of the gas-phase reaction between S4 and CF3OF. This reaction obeys the rate expression r=k(SF4)(CF3OF) The following mechanism has been
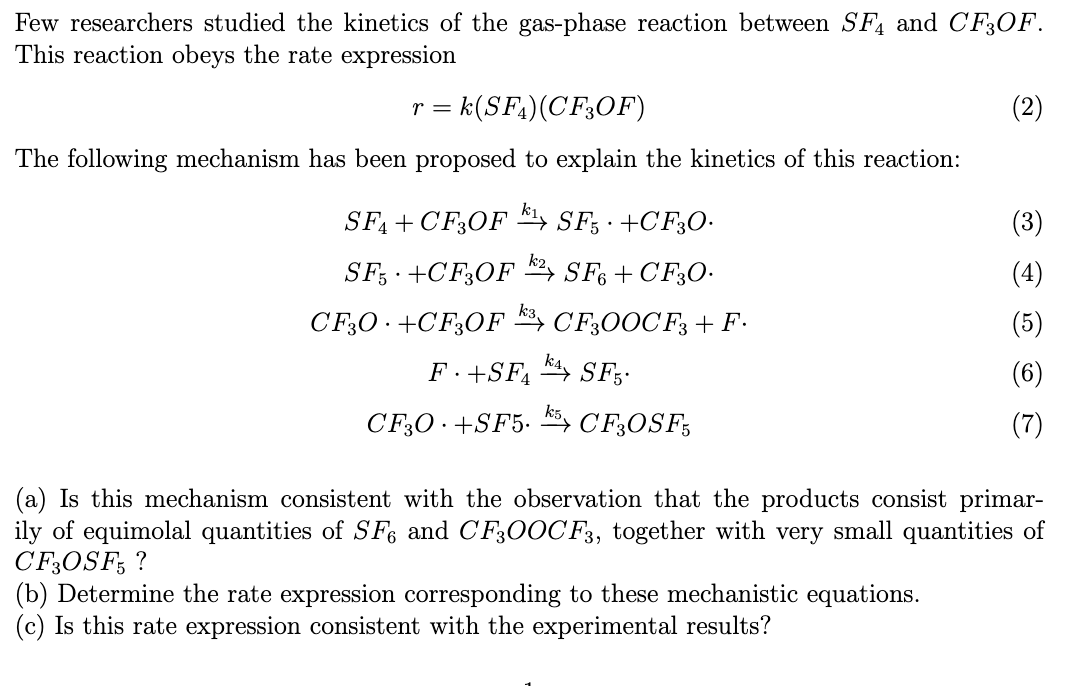 Few researchers studied the kinetics of the gas-phase reaction between S4 and CF3OF. This reaction obeys the rate expression r=k(SF4)(CF3OF) The following mechanism has been proposed to explain the kinetics of this reaction: SF4+CF3OFk1SF5+CF3O.SF5+CF3OFk2SF6+CF3O.CF3O+CF3OFk3CF3OOCF3+F.F+SF4k4SF5.CF3O+SF5k5CF3OSF5 (a) Is this mechanism consistent with the observation that the products consist primarily of equimolal quantities of SF6 and CF3OOCF3, together with very small quantities of CF3OSF5? (b) Determine the rate expression corresponding to these mechanistic equations. (c) Is this rate expression consistent with the experimental results
Few researchers studied the kinetics of the gas-phase reaction between S4 and CF3OF. This reaction obeys the rate expression r=k(SF4)(CF3OF) The following mechanism has been proposed to explain the kinetics of this reaction: SF4+CF3OFk1SF5+CF3O.SF5+CF3OFk2SF6+CF3O.CF3O+CF3OFk3CF3OOCF3+F.F+SF4k4SF5.CF3O+SF5k5CF3OSF5 (a) Is this mechanism consistent with the observation that the products consist primarily of equimolal quantities of SF6 and CF3OOCF3, together with very small quantities of CF3OSF5? (b) Determine the rate expression corresponding to these mechanistic equations. (c) Is this rate expression consistent with the experimental results Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


