Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Figure 1 shows the elementary reactions of ethylene oxide in series take place in a continuous constant-flow stirred tank reactor (CSTR) under isothermal conditions. The
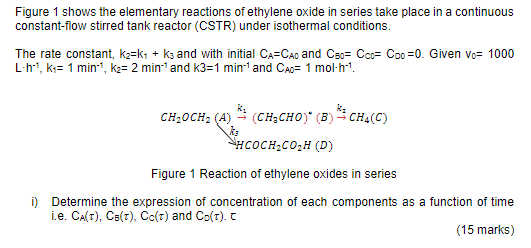
Figure 1 shows the elementary reactions of ethylene oxide in series take place in a continuous constant-flow stirred tank reactor (CSTR) under isothermal conditions. The rate constant, kaks + ks and with initial Ca=Cao and C5o= Coo= Coo=0. Given Vo= 1000 L'h'', k = 1 min", ka= 2 min' and K3=1 min-1 and Cao= 1 mol-h!. CH20CH, (A) (CH2CHO) (B) CH(C) NHCOCH.CO2H (D) Figure 1 Reaction of ethylene oxides in series i) Determine the expression of concentration of each components as a function of time i.e. CA(T), C(t), Co(t) and Co(t). C (15 marks)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


