Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Figure 8.A-14 shows a Rankine steam cycle in which a double-extraction turbine is used to supply both power and process steam for an industrial
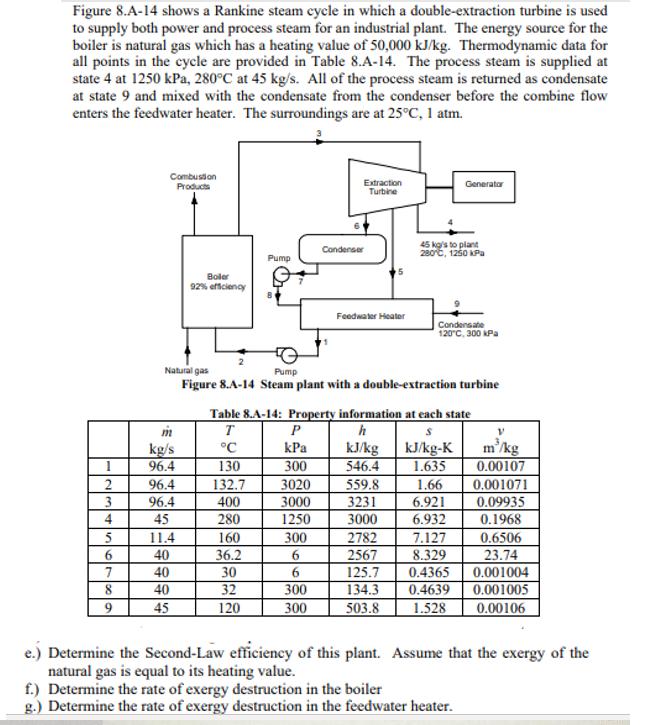
Figure 8.A-14 shows a Rankine steam cycle in which a double-extraction turbine is used to supply both power and process steam for an industrial plant. The energy source for the boiler is natural gas which has a heating value of 50,000 kJ/kg. Thermodynamic data for all points in the cycle are provided in Table 8.A-14. The process steam is supplied at state 4 at 1250 kPa, 280C at 45 kg/s. All of the process steam is returned as condensate at state 9 and mixed with the condensate from the condenser before the combine flow enters the feedwater heater. The surroundings are at 25C, 1 atm. 2 3 4 5 6 8 9 Combustion Products m kg/s 96.4 96.4 96.4 45 11.4 40 Boler 92% efficiency 40 40 45 Pump 130 132.7 400 280 160 36.2 30 32 120 Natural gas Pump Figure 8.A-14 Steam plant with a double-extraction turbine Extraction Turbine P kPa 300 3020 3000 1250 Condenser 300 6 6 300 300 Feedwater Heater Table 8.A-14: Property information at each state T h S C kJ/kg-K 1.635 1.66 6.921 6.932 kJ/kg 546.4 45 kg/s to plant 280C, 1250 kPa 559.8 3231 3000 2782 2567 125.7 Generator Condensate 120C, 300 kPa 7.127 8.329 0.4365 134.3 0.4639 503.8 1.528 f.) Determine the rate of exergy destruction in the boiler g.) Determine the rate of exergy destruction in the feedwater heater. V m'/kg 0.00107 0.001071 0.09935 0.1968 0.6506 23.74 0.001004 0.001005 0.00106 e.) Determine the Second-Law efficiency of this plant. Assume that the exergy of the natural gas is equal to its heating value.
Step by Step Solution
There are 3 Steps involved in it
Step: 1
Solutions Turbines is adiabatic Applying steady flow energy equation is turbine for generator power ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


