Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Fill in the following table. Make sure to add appropriate units to the columns. (5 marks) Table 2. Absorbance of different concentrations of CuSO4 solutions.
Fill in the following table. Make sure to add appropriate units to the columns. (5 marks)
Table 2. Absorbance of different concentrations of CuSO4 solutions.
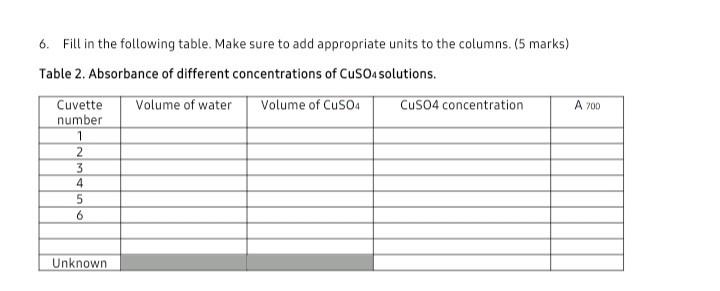

6. Fill in the following table. Make sure to add appropriate units to the columns. (5 marks) Table 2. Absorbance of different concentrations of CuSO4 solutions. NOTES: 1. Wherever you have a number, you must have a unit associated with that number (an exception is absorbance values, which reflect unit-less ratios). In a table, there is normally a separate heading for each column that includes the units, so the units don't have to be repeated with each individual value. 2. Absorbance must always be associated with a wavelength (e.g. Asso). PART 1 - DILUTION SERIES FOR A COLOURED SOLUTION 1. Fill in the following table. Make sure to add appropriate units to the columns. (4 marks) Table 1. Absorbance of blue coloured solutions at different concentrations. 2. In the space below, show a sample calculation for the concentration of the blue coloured solution in Cuvette \#4. (2 marks) 3. Using the blue coloured solution concentration and absorbance data from Table 1 above, create a properly formatted standard curve and paste into the space below. Refer back to the formatting trendline and include the equation of the line on the graph. ( 7 marks) BIOLOGY 126 - PHYSIOLOGICAL BASIS OF LIFE LAB 1 - STANDARD CURVE PART 2 - DETERMINING CONCENTRATION OF A COPPER SULPHATE SOLUTION 4. Calculate the molarity of your copper sulphate solution. Show your work! (2 marks) 5. Show the calculation for how you prepared 30ml of a 0.1M copper sulphate solution. (1 mark) 6. Fill in the following table. Make sure to add appropriate units to the columns. (5 marks) Table 2. Absorbance of different concentrations of CuSO4 solutions. 7. In the space below, show a sample calculation for the concentration of CuSO4 in Cuvette \#2. (2 marks) 8. Using the CuSOs solution concentration and absorbance data from Table 2 above, create a properly formatted standard curve and paste into the space below. Refer back to the formatting requirements from the Lab introduction prior to submitting your graph. Also, be sure to add a trendline and include the equation of the line on the graph. (7 marks) 9. Use the equation of the trendline to calculate the concentration of the unknown copper sulphate solution. Show your work and final answer in the space below. (2 marks). 6. Fill in the following table. Make sure to add appropriate units to the columns. (5 marks) Table 2. Absorbance of different concentrations of CuSO4 solutions. NOTES: 1. Wherever you have a number, you must have a unit associated with that number (an exception is absorbance values, which reflect unit-less ratios). In a table, there is normally a separate heading for each column that includes the units, so the units don't have to be repeated with each individual value. 2. Absorbance must always be associated with a wavelength (e.g. Asso). PART 1 - DILUTION SERIES FOR A COLOURED SOLUTION 1. Fill in the following table. Make sure to add appropriate units to the columns. (4 marks) Table 1. Absorbance of blue coloured solutions at different concentrations. 2. In the space below, show a sample calculation for the concentration of the blue coloured solution in Cuvette \#4. (2 marks) 3. Using the blue coloured solution concentration and absorbance data from Table 1 above, create a properly formatted standard curve and paste into the space below. Refer back to the formatting trendline and include the equation of the line on the graph. ( 7 marks) BIOLOGY 126 - PHYSIOLOGICAL BASIS OF LIFE LAB 1 - STANDARD CURVE PART 2 - DETERMINING CONCENTRATION OF A COPPER SULPHATE SOLUTION 4. Calculate the molarity of your copper sulphate solution. Show your work! (2 marks) 5. Show the calculation for how you prepared 30ml of a 0.1M copper sulphate solution. (1 mark) 6. Fill in the following table. Make sure to add appropriate units to the columns. (5 marks) Table 2. Absorbance of different concentrations of CuSO4 solutions. 7. In the space below, show a sample calculation for the concentration of CuSO4 in Cuvette \#2. (2 marks) 8. Using the CuSOs solution concentration and absorbance data from Table 2 above, create a properly formatted standard curve and paste into the space below. Refer back to the formatting requirements from the Lab introduction prior to submitting your graph. Also, be sure to add a trendline and include the equation of the line on the graph. (7 marks) 9. Use the equation of the trendline to calculate the concentration of the unknown copper sulphate solution. Show your work and final answer in the space below. (2 marks)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


