Answered step by step
Verified Expert Solution
Question
1 Approved Answer
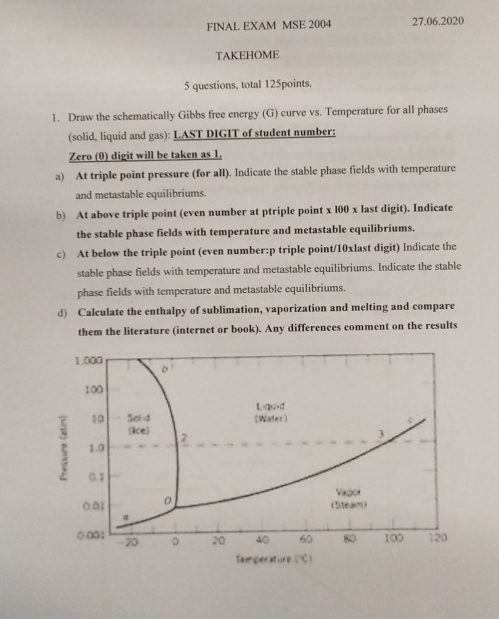
FINAL EXAM MSE 2 0 0 4 2 7 . 0 6 . 2 0 2 0 TAKEHOME 5 questions, total 1 2 5 points.
FINAL EXAM MSE
TAKEHOME
questions, total points.
Draw the schematically Gibbs free energy curve vs Temperature for all phases solid liquid and gas: LAST DIGIT of student number:
Zero digit will be taken as
a At triple point pressure for all Indicate the stable phase fields with temperature and metastable equilibriums.
b At above triple point even number at ptriple point last digit Indicate the stable phase fields with temperature and metastable equilibriums.
c At below the triple point even number:p triple pointxlast digit Indicate the stable phase fields with temperature and metastable equilibriums. Indicate the stable phase fields with temperature and metastable equilibriums.
d Calculate the enthalpy of sublimation, vaporization and melting and compare them the literature internet or book Any differences comment on the results
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


