Answered step by step
Verified Expert Solution
Question
1 Approved Answer
DO (vi) 1. Provide structure(s) the major organic product(s) of each of the following reactions or sequences of reactions. Show all relevant stereochemistry, if
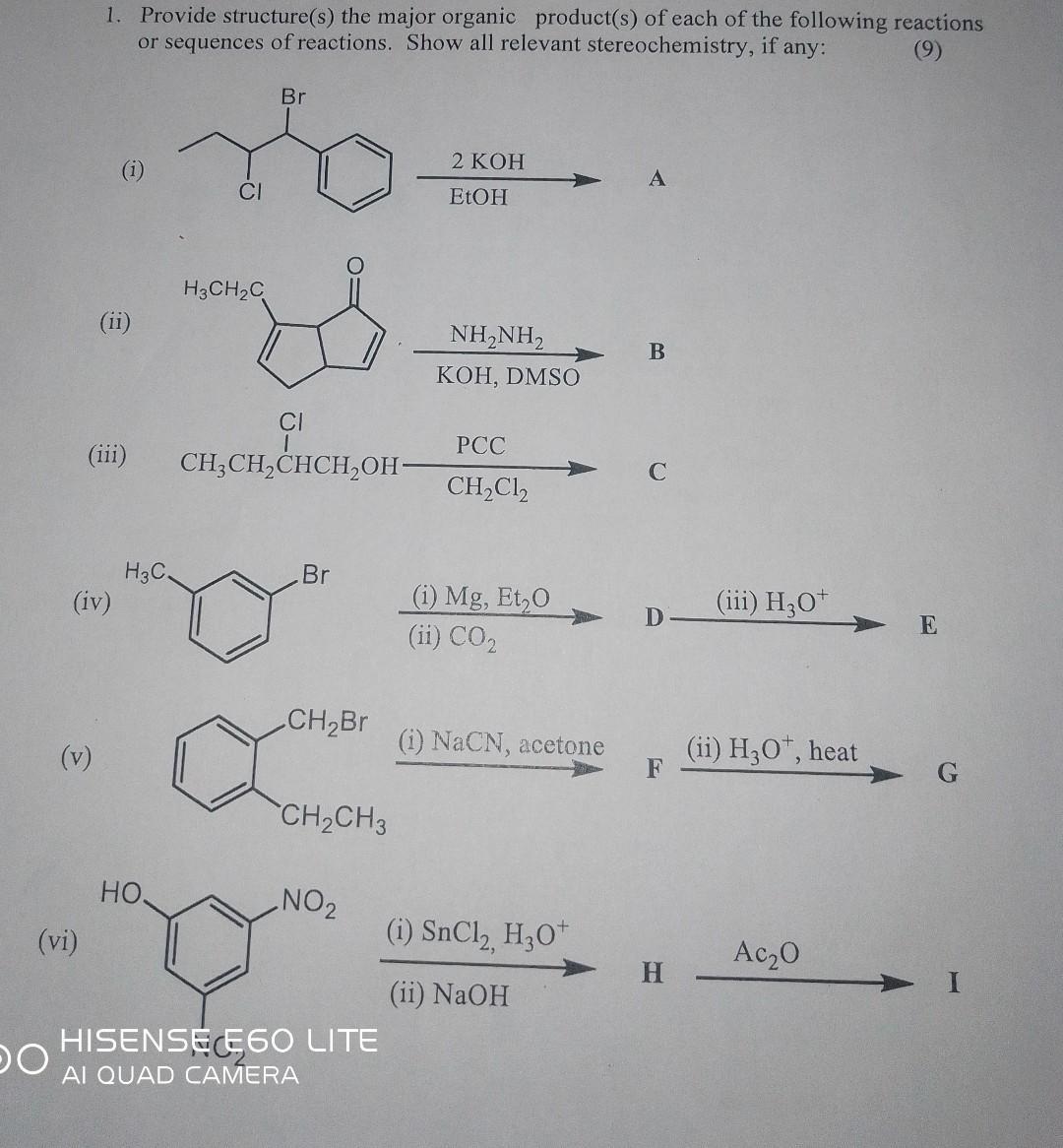
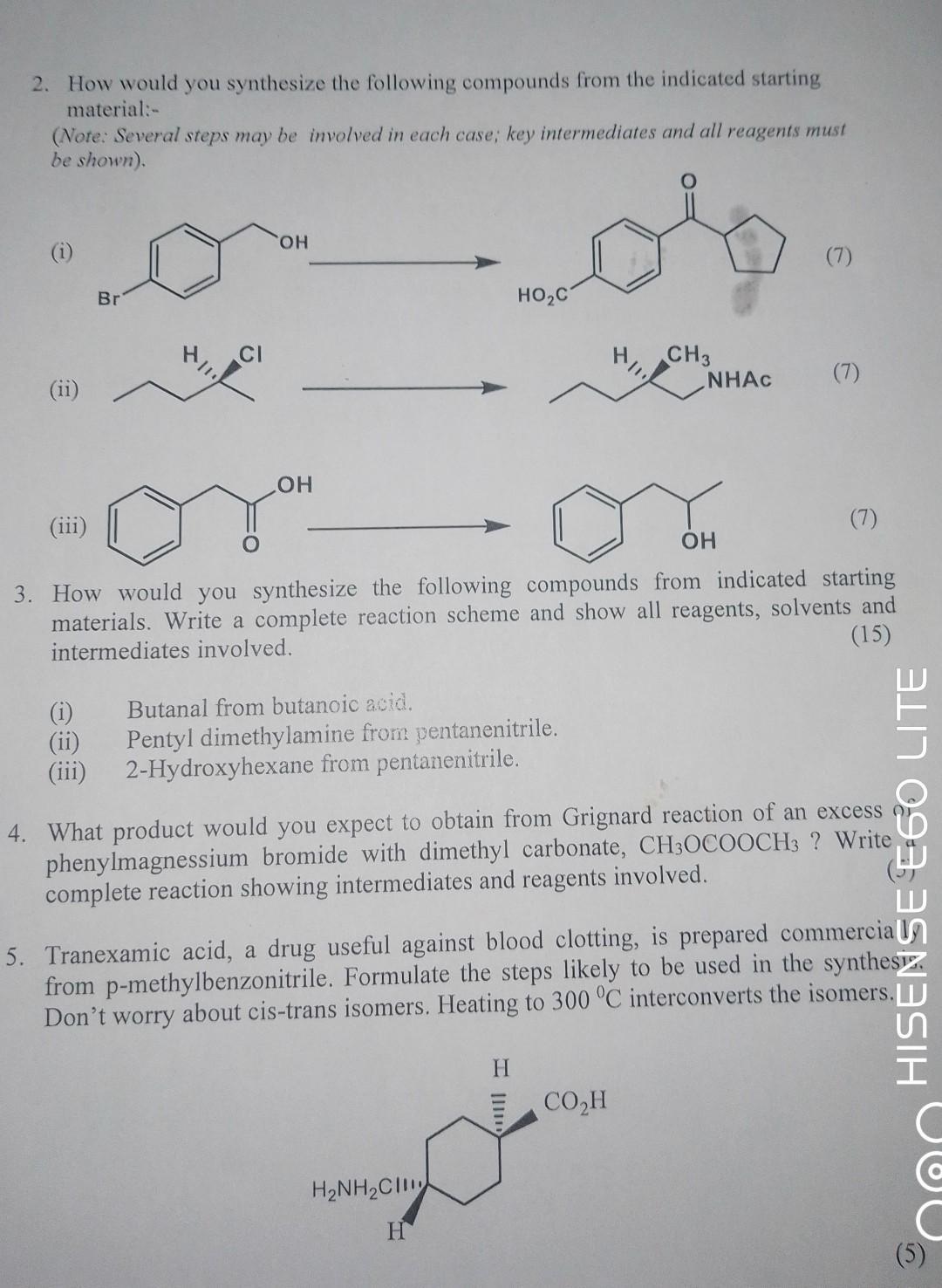
DO (vi) 1. Provide structure(s) the major organic product(s) of each of the following reactions or sequences of reactions. Show all relevant stereochemistry, if any: (9) (iii) (iv) (v) H3C. , CI H3CHC Br CI CH3CHCHCHOH- Br CHBr CHCH3 NO HISENSE E60 LITE AI QUAD CAMERA 2 KOH EtOH NH,NH, KOH, DMSO PCC CHCl (i) Mg, Et0 (ii) CO (i) NaCN, acetone (i) SnCl, H3O+ (ii) NaOH A B C D F H (iii) H3O+ (ii) H3O+, heat Ac0 E G 2. How would you synthesize the following compounds from the indicated starting material:- (Note: Several steps may be involved in each case; key intermediates and all reagents must be shown). (ii) Br (i) (ii) (iii) CI OH OH HOC Butanal from butanoic acid. Pentyl dimethylamine from pentanenitrile. 2-Hydroxyhexane from pentanenitrile. H/ HNH, CH H CH3 (iii) (7) OH 3. How would you synthesize the following compounds from indicated starting materials. Write a complete reaction scheme and show all reagents, solvents and intermediates involved. (15) NHAC COH (7) (7) 4. What product would you expect to obtain from Grignard reaction of an excess phenylmagnessium bromide with dimethyl carbonate, CH3OCOOCH3 ? Write complete reaction showing intermediates and reagents involved. prepared commercia 5. Tranexamic acid, a drug useful against blood clotting, from p-methylbenzonitrile. Formulate the steps likely to be used in the synthesiz Don't worry about cis-trans isomers. Heating to 300 C interconverts the isomers. HISENSE E60 LITE C C (5)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


