Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Focus on questions 1 and 2. Elementary Principles of Chemical Processes 1. What is the change in internal energy when 30kg mol of air are
Focus on questions 1 and 2. Elementary Principles of Chemical Processes
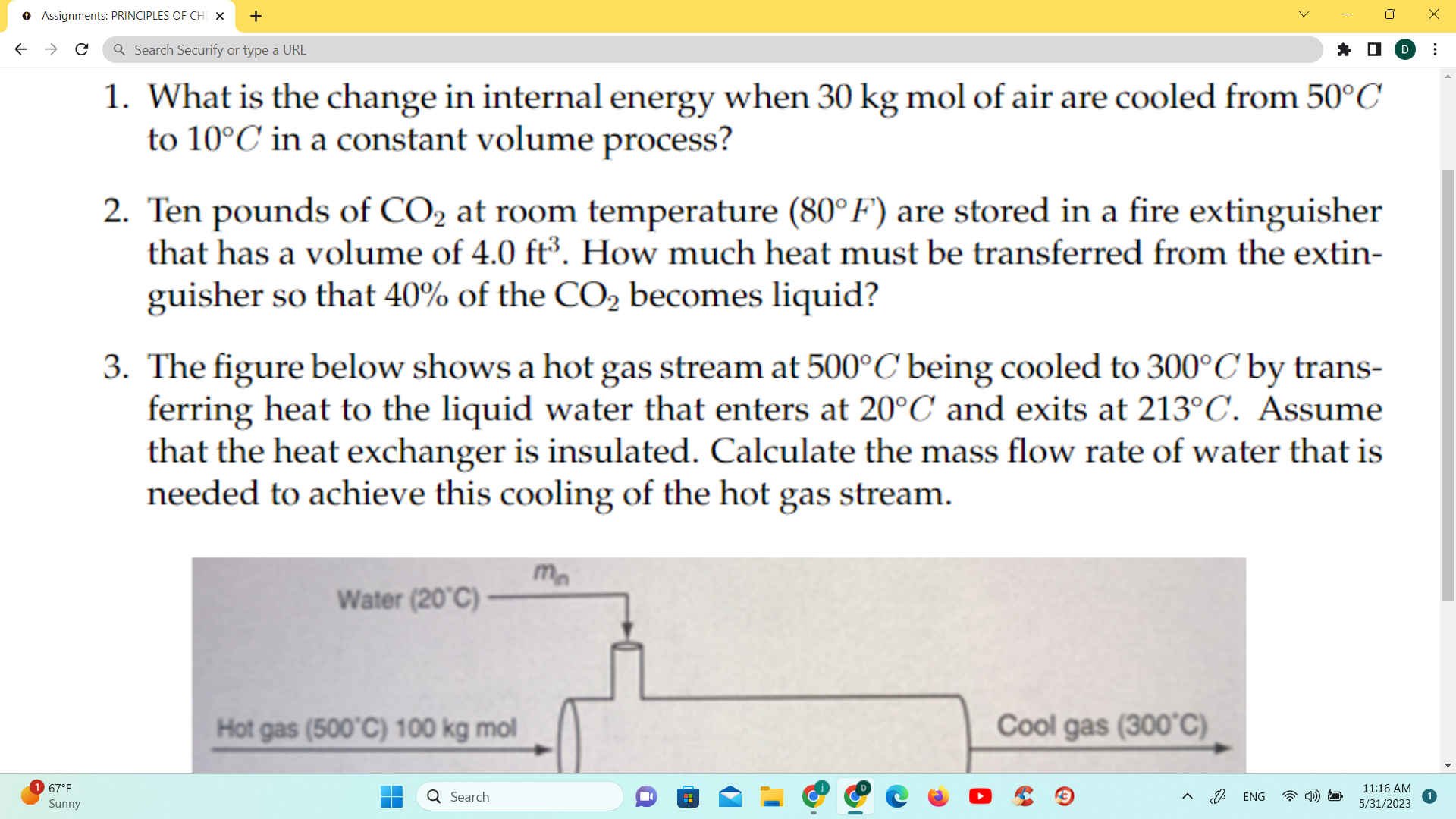 1. What is the change in internal energy when 30kg mol of air are cooled from 50C to 10C in a constant volume process? 2. Ten pounds of CO2 at room temperature (80F) are stored in a fire extinguisher that has a volume of 4.0ft3. How much heat must be transferred from the extinguisher so that 40% of the CO2 becomes liquid? 3. The figure below shows a hot gas stream at 500C being cooled to 300C by transferring heat to the liquid water that enters at 20C and exits at 213C. Assume that the heat exchanger is insulated. Calculate the mass flow rate of water that is needed to achieve this cooling of the hot gas stream
1. What is the change in internal energy when 30kg mol of air are cooled from 50C to 10C in a constant volume process? 2. Ten pounds of CO2 at room temperature (80F) are stored in a fire extinguisher that has a volume of 4.0ft3. How much heat must be transferred from the extinguisher so that 40% of the CO2 becomes liquid? 3. The figure below shows a hot gas stream at 500C being cooled to 300C by transferring heat to the liquid water that enters at 20C and exits at 213C. Assume that the heat exchanger is insulated. Calculate the mass flow rate of water that is needed to achieve this cooling of the hot gas stream Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


