Answered step by step
Verified Expert Solution
Question
1 Approved Answer
For a reaction. A = P, the plots of [A] and [P] with time at temperatures T, and T, are given below. [JEE 2018] 10-
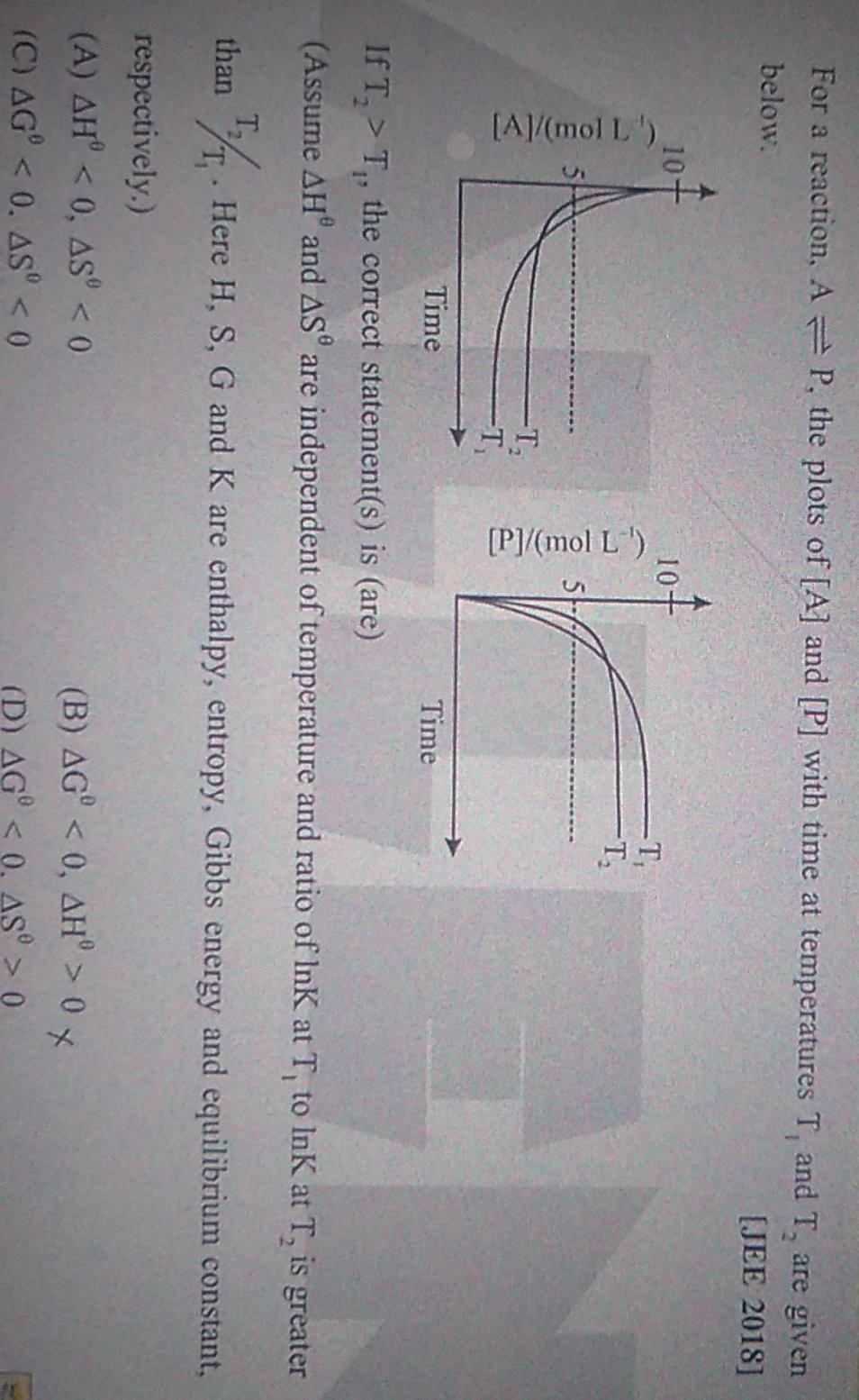
For a reaction. A = P, the plots of [A] and [P] with time at temperatures T, and T, are given below. [JEE 2018] 10- 10- -T -T [Al/mol L ) [P]/(mol L') T, -T Time Time IfT, > T, the correct statement(s) is (are) (Assume AHand AS are independent of temperature and ratio of InK at T to Ink at T, is greater than 17. Here H, S, G and K are enthalpy, entropy, Gibbs energy and equilibrium constant , respectively.) (A) AH 0 X (D) AG
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


