Answered step by step
Verified Expert Solution
Question
1 Approved Answer
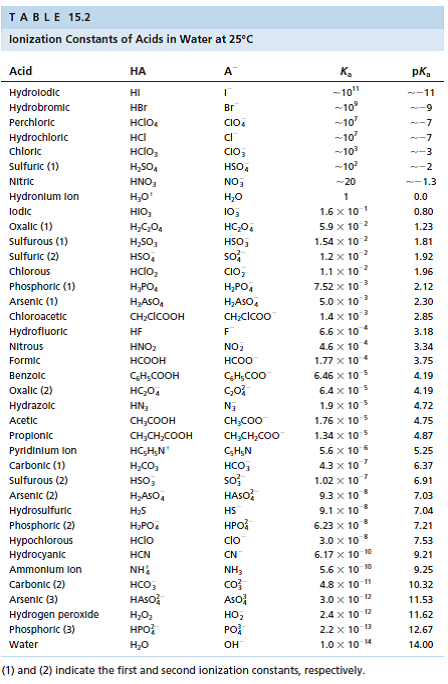
For each of the following acids (Use Table 15.2 in Oxtoby as necessary), A) Perchloric, HClO 4 B) Chloroacetic, CH 2 ClCOOH C) Hydrogen Peroxide,
For each of the following acids (Use Table 15.2 in Oxtoby as necessary),
A) Perchloric, HClO4
B) Chloroacetic, CH2ClCOOH
C) Hydrogen Peroxide, H2O2
provide the following information:
i) identify them as strong or weak
ii) Write the hydrolysis reaction
iii) Calculate the pKa for this chemical
iv)Calculate the pKb for the conjugate species of this chemical
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


