Answered step by step
Verified Expert Solution
Question
1 Approved Answer
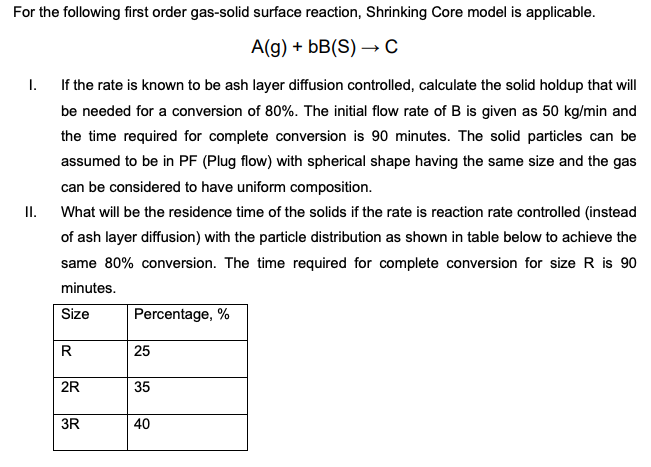
For the following first order gas - solid surface reaction, Shrinking Core model is applicable. A ( g ) + b B ( S )
For the following first order gassolid surface reaction, Shrinking Core model is applicable.
I. If the rate is known to be ash layer diffusion controlled, calculate the solid holdup that will
be needed for a conversion of The initial flow rate of is given as and
the time required for complete conversion is minutes. The solid particles can be
assumed to be in PF Plug flow with spherical shape having the same size and the gas
can be considered to have uniform composition.
II What will be the residence time of the solids if the rate is reaction rate controlled instead
of ash layer diffusion with the particle distribution as shown in table below to achieve the
same conversion. The time required for complete conversion for size is
minutes.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


