Question
for the following reaction, how do you find how many electrons transferred? i understand you must write out and balance the half reactions but i
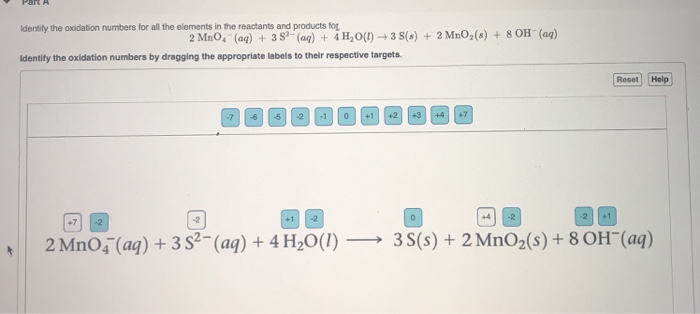
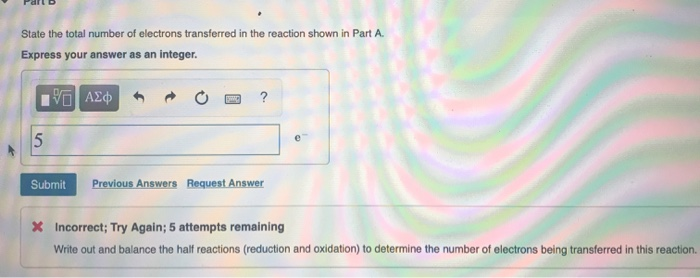
Identify the oxidation numbers for all the elements in the reactants and products for 2 MnO (aq) + 382 (aq) + 4HO(1)3 S(s) + 2 MnO (s) + 8 OH(aq) Identify the oxidation numbers by dragging the appropriate labels to their respective targets. -7 -5 -2 -1 0 +1 +2 +3 - +4 +7 Reset Help +1 -2 0 -2 2 MnO4 (aq) + 3 S2- (aq) + 4HO(1) 3S(s) + 2 MnO (s) + 8 OH(aq)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Fundamentals of Cost Accounting
Authors: William Lanen, Shannon Anderson, Michael Maher
3rd Edition
9780078025525, 9780077517359, 77517350, 978-0077398194
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



