Answered step by step
Verified Expert Solution
Question
1 Approved Answer
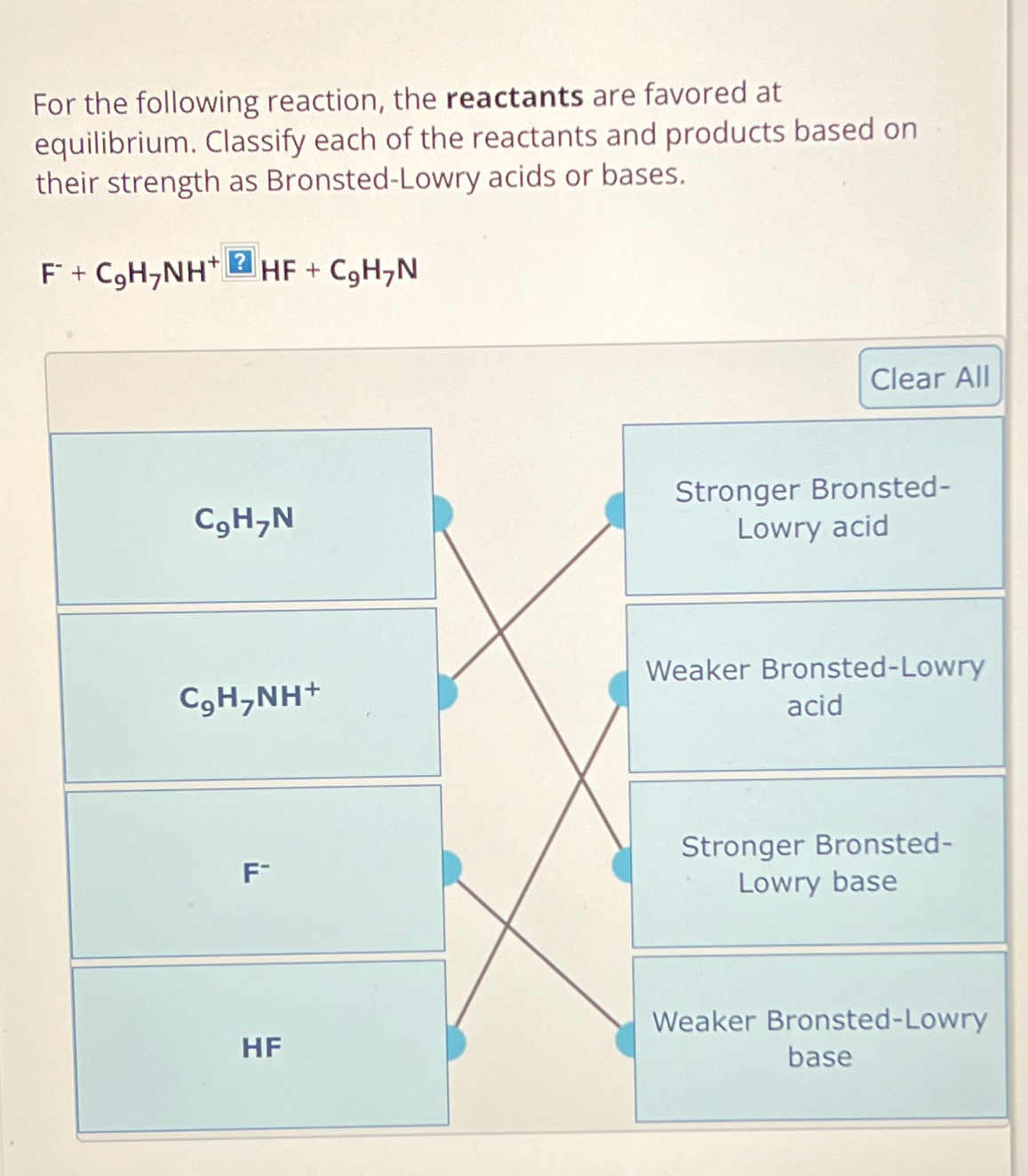
For the following reaction, the reactants are favored at equilibrium. Classify each of the reactants and products based on their strength as Bronsted - Lowry
For the following reaction, the reactants are favored at equilibrium. Classify each of the reactants and products based on their strength as BronstedLowry acids or bases.
Says that my answer is wrong please help
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


