Answered step by step
Verified Expert Solution
Question
1 Approved Answer
For the reaction: EO+ WEG The rate law is: r= (0.1113 Peo Pw)/(1+0.475Peo+0.322Pw+0.414PEG 12 Neglecting pressure drop, calculate the catalyst weight necessary to achieve 80%
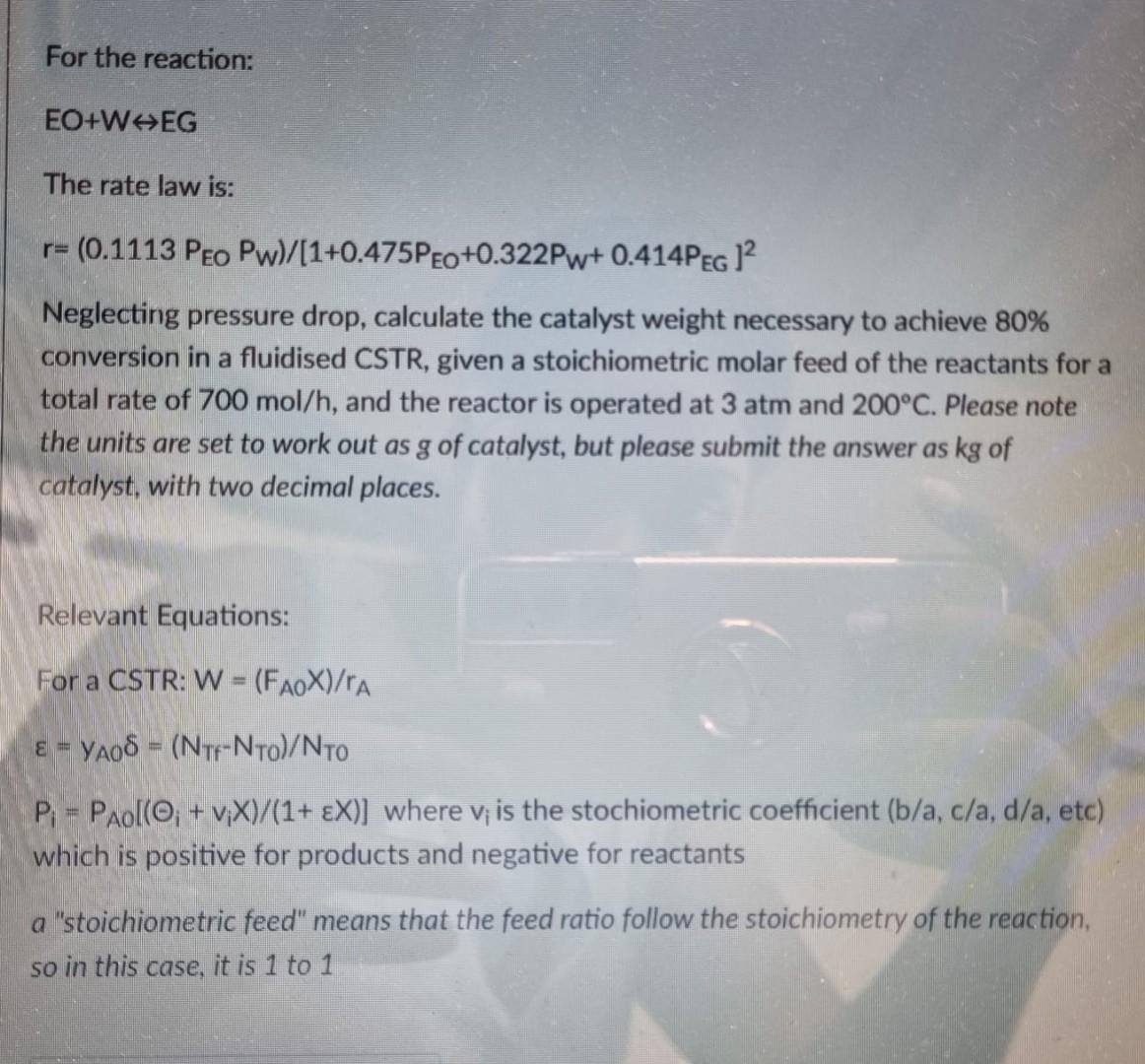
For the reaction: EO+ WEG The rate law is: r= (0.1113 Peo Pw)/(1+0.475Peo+0.322Pw+0.414PEG 12 Neglecting pressure drop, calculate the catalyst weight necessary to achieve 80% conversion in a fluidised CSTR, given a stoichiometric molar feed of the reactants for a total rate of 700 mol/h, and the reactor is operated at 3 atm and 200C. Please note the units are set to work out as g of catalyst, but please submit the answer as kg of catalyst, with two decimal places. Relevant Equations: For a CSTR: W = (FAOX)/rA = YA08 = (Ntf-Nro)/NTO P = Paol(O; + vX)/(1+ X)] where v; is the stochiometric coefficient (b/a, c/a, d/a, etc) which is positive for products and negative for reactants a "stoichiometric feed" means that the feed ratio follow the stoichiometry of the reaction, so in this case, it is 1 to 1
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


