Question
For the response: Given: The molar mass of A is 84 g mol. Show calculations. A. Calculate the activation energy for the reaction B. Determine
For the response: 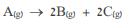 Given:
Given:
The molar mass of A is 84 g mol. Show calculations.
A. Calculate the activation energy for the reaction
B. Determine the order of response based on the data. Explain.
C. In an experiment performed at a temperature of 300 C, gas A was introduced into a 60.0 hard vessel liter. After 00.3 minutes from the start of the reaction it was found that the concentration of B increased to 80mM.6. i. Calculate the gas concentration A in a time of 00.3 minutes. Show calculations. ii. Calculate the partial pressure of gas A in a time of 00.3 minutes. Show calculations. iii. Does the half-life increase / decrease / do not change when the temperature rises? Explain. iv. Calculate the rate constant at a temperature of 50 C. Show calculations. v. How long from the beginning of the reaction will the concentration of the gas A equal to the concentration of the gas? C? Show calculations
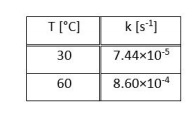
Alg) 2B + 2Cg T[C] k [s] 30 7.44x10-5 60 8.60x104Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


