Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Formaldehyde (CH20) is produced industrially by the catalytic oxidation of methanol (CH3OH) according to the following reaction: CH3OH + 1/202 CH2O + H2O Unfortunately, under
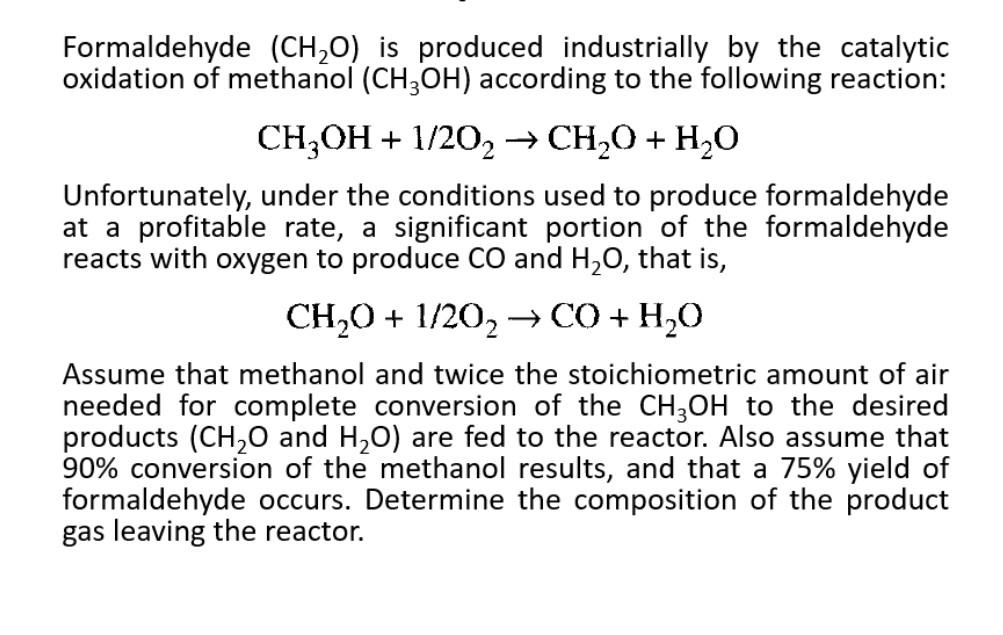
Formaldehyde (CH20) is produced industrially by the catalytic oxidation of methanol (CH3OH) according to the following reaction: CH3OH + 1/202 CH2O + H2O Unfortunately, under the conditions used to produce formaldehyde at a profitable rate, a significant portion of the formaldehyde reacts with oxygen to produce CO and H20, that is, CH,0 + 1/202C0+4,0 H20 Assume that methanol and twice the stoichiometric amount of air needed for complete conversion of the CH3OH to the desired products (CH20 and H20) are fed to the reactor. Also assume that 90% conversion of the methanol results, and that a 75% yield of formaldehyde occurs. Determine the composition of the product gas leaving the reactor
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


