Answered step by step
Verified Expert Solution
Question
1 Approved Answer
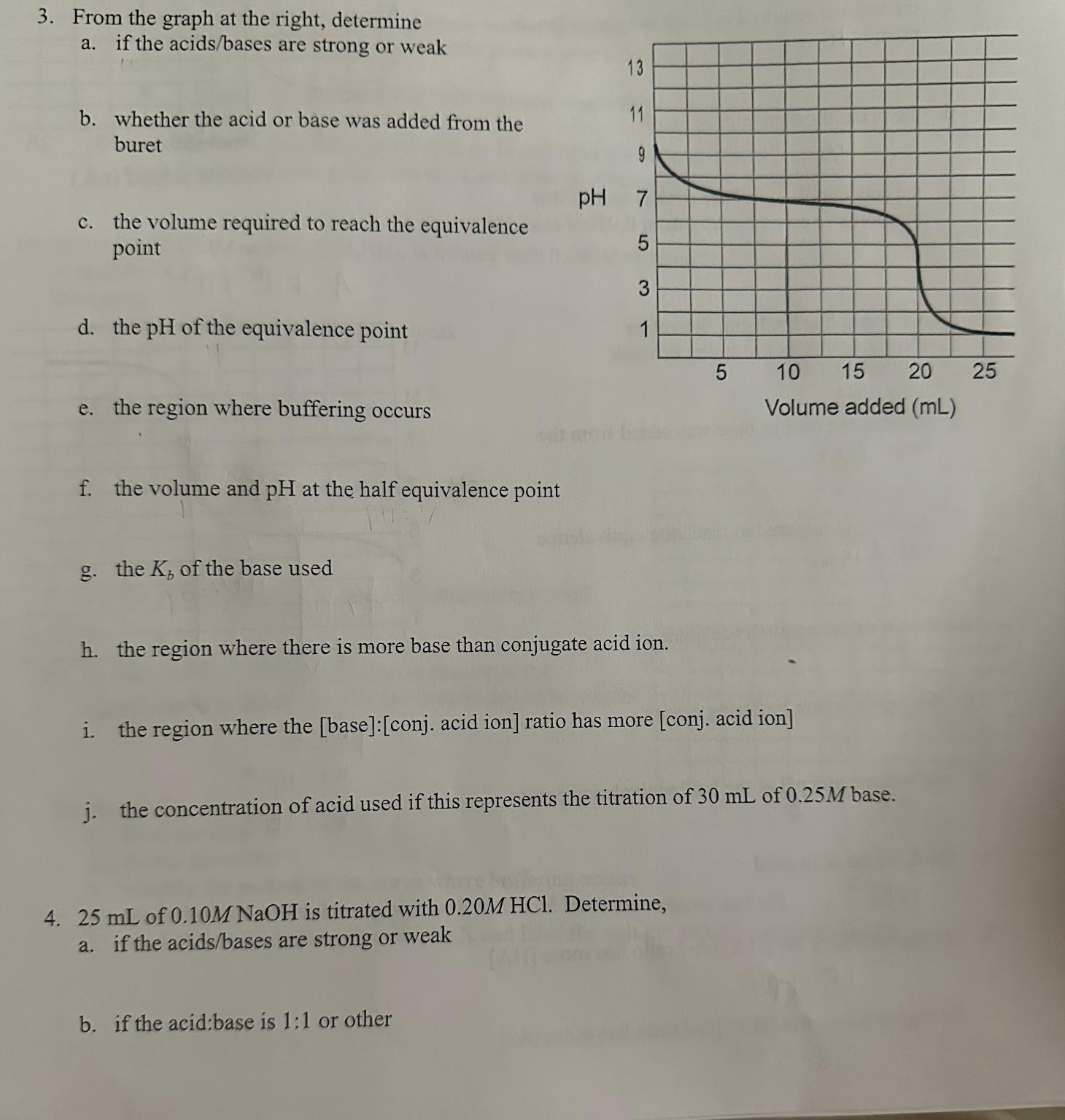
From the graph at the right, determine a . if the acids / bases are strong or weak b . whether the acid or base
From the graph at the right, determine
a if the acidsbases are strong or weak
b whether the acid or base was added from the buret
c the volume required to reach the equivalence point
d the of the equivalence point
e the region where buffering occurs
f the volume and at the half equivalence point
g the of the base used
h the region where there is more base than conjugate acid ion.
i the region where the base:conj acid ion ratio has more conj acid ion
j the concentration of acid used if this represents the titration of of base.
of MNaOH is titrated with Determine,
a if the acidsbases are strong or weak
b if the acid:base is : or other
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


