Answered step by step
Verified Expert Solution
Question
1 Approved Answer
How many moles of zinc are in 3.80kg of zinc? Express your answer to three significant figures and include the appropriate units. Learning Goal: To
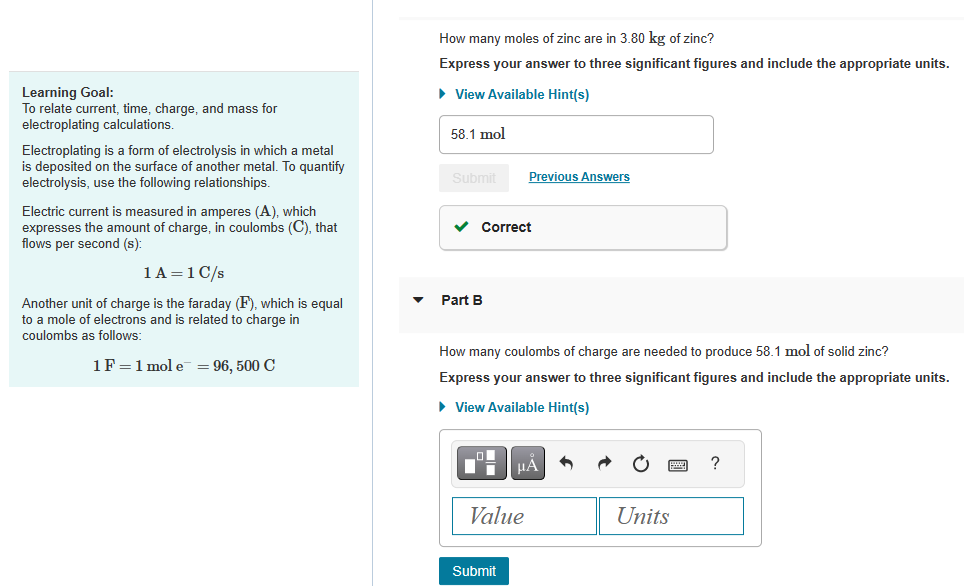 How many moles of zinc are in 3.80kg of zinc? Express your answer to three significant figures and include the appropriate units. Learning Goal: To relate current, time, charge, and mass for electroplating calculations. Electroplating is a form of electrolysis in which a metal is deposited on the surface of another metal. To quantify electrolysis, use the following relationships. Electric current is measured in amperes (A), which expresses the amount of charge, in coulombs (C), that flows per second (s): 1A=1C/s Another unit of charge is the faraday (F), which is equal Part B to a mole of electrons and is related to charge in coulombs as follows: How many coulombs of charge are needed to produce 58.1mol of solid zinc? 1F=1mole=96,500C Express your answer to three significant figures and include the appropriate units
How many moles of zinc are in 3.80kg of zinc? Express your answer to three significant figures and include the appropriate units. Learning Goal: To relate current, time, charge, and mass for electroplating calculations. Electroplating is a form of electrolysis in which a metal is deposited on the surface of another metal. To quantify electrolysis, use the following relationships. Electric current is measured in amperes (A), which expresses the amount of charge, in coulombs (C), that flows per second (s): 1A=1C/s Another unit of charge is the faraday (F), which is equal Part B to a mole of electrons and is related to charge in coulombs as follows: How many coulombs of charge are needed to produce 58.1mol of solid zinc? 1F=1mole=96,500C Express your answer to three significant figures and include the appropriate units Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


